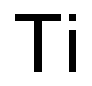티타늄
|
|
티타늄 속성
- 녹는점
- 1660 °C (lit.)
- 끓는 점
- 3287 °C (lit.)
- 밀도
- 4.5 g/mL at 25 °C (lit.)
- 벌크 밀도
- 1260kg/m3
- 인화점
- 0°C
- 저장 조건
- no restrictions.
- 물리적 상태
- 철사
- 색상
- 은회색
- Specific Gravity
- 4.5
- 수소이온지수(pH)
- 5-8 (50g/L in H2O, suspension)
- Flame Color
- Silver-white
- 비저항
- 42.0 μΩ-cm, 20°C
- 수용성
- 물에 불용성.
- Merck
- 13,9547
- 노출 한도
- ACGIH: TWA 2.5 mg/m3
NIOSH: IDLH 250 mg/m3
- 안정성
- 안정적인. 먼지는 자연적으로 가연성이 있는 것으로 생각되며 공기와 폭발성 혼합물을 형성할 수 있습니다. 가연성 고체. 무기산, 할로겐, 이산화탄소, 강산화제와 호환되지 않습니다.
- InChIKey
- RTAQQCXQSZGOHL-UHFFFAOYSA-N
- CAS 데이터베이스
- 7440-32-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/21/22-11-17-36/38 | ||
| 안전지침서 | 16-36/37/39-33-27-26-6-43 | ||
| 유엔번호(UN No.) | UN 2878 4.1/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | XR1700000 | ||
| F 고인화성물질 | 10 | ||
| 자연 발화 온도 | 860 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 4.2 | ||
| 포장분류 | III | ||
| HS 번호 | 81089020 | ||
| 유해 물질 데이터 | 7440-32-6(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-33881 |
티타늄 C화학적 특성, 용도, 생산
개요
Titanium was discovered by the Reverend William Gregor in 1791, and is named after the ‘Titans’ of Greek mythology. The metal was not isolated in a pure state until 1910, and useful quantities were not available for industrial applications until 1946, when an economical purification process was developed.화학적 성질
Titanium is a silvery metal or dry, dark-gray amorphous, lustrous powder.물리적 성질
Positioned at the top of group 4 (IVB), titanium heads up a group of metals sometimesreferred to as the “titanium group.” Members of this group have some similar properties.Titanium’s density is 4.5 g/cm3, which makes it heavier than aluminum but not as heavy asiron. Its melting point is high at 1,660°C, and its boiling point is even higher at 3287°C.Titanium metal is harder than steel but much lighter and does not corrode in seawater,which makes it an excellent alloy metal for use in most environmental conditions. It is alsoparamagnetic, which means that it is not responsive to magnetic fields. It is not a very goodconductor of heat or electricity.Isotopes
There are 23 known isotopes of titanium. All but five are radioactive, rangingfrom Ti-38 to Ti-61, and have half-lives varying from a few nanoseconds to a few hours.The percentages of the five stable isotopes found in nature are as follows: 46Ti = 8.25%,47Ti = 7.44%, 48Ti = 73.72%, 49Ti = 5.41%, and 50Ti = 5.18%.Origin of Name
It was named after “Titans,” meaning the first sons of the Earth as stated in Greek mythology.출처
Titanium is the ninth most abundant element found in the Earth’s crust, but not in pureform. It is found in two minerals: rutile, which is titanium dioxide (TiO2), and ilmenite(FeTiO3). It is also found in some iron ores and in the slag resulting from the productionof iron. The mineral rutile is the major source of titanium production in the United States.Although titanium is widely spread over the crust of the Earth, high concentrations of itsminerals are scarce. In the past it was separated from it ores by an expensive process ofchemical reduction that actually limited the amount of metal produced. A two-step processinvolves heating rutile with carbon and chlorine to produce titanium tetrachloride—TiO2+ C + 2Cl2 ?→ TiCl4 + CO2—which is followed by heating the titanium tetrachloridewith magnesium in an inert atmosphere: TiCl4 + 2Mg ?→ Ti + 2 MgCl2. As recently as theyear 2000, a method of electrolysis was developed using titanium tetrachloride in a bath ofrare-earth salts. This process can be used on a commercial scale that makes the productionof titanium much less expensive. Titanium was, and still is, a difficult element to extractfrom its ore.Titanium is found throughout the universe and in the stars, the sun, the moon, and themeteorites that land on Earth.Characteristics
As the first element in group 4, titanium has characteristics similar to those of the othermembers of this group: Zr, Hf, and Rf. Titanium is a shiny, gray, malleable, and ductile metalcapable of being worked into various forms and drawn into wires.역사
In 1791 Reverend William Gregor (1761–1817), an amateur mineralogist, discoveredan odd black sandy substance in his neighborhood. Because it was somewhat magnetic, hecalculated that it was almost 50% magnetite (a form of iron ore). Most of the remainder ofthe sample was a reddish-brown powder he dissolved in acid to produce a yellow substance.Thinking he had discovered a new mineral, he named it “menachanite,” after the Menachanregion in Cornwall where he lived. During this period, Franz Joseph Muller (1740–1825) alsoproduced a similar substance that he could not identify. In 1793 Martin Heinrich Klaproth(1743–1817), who discovered several new elements and is considered the father of modernanalytical chemistry, identified the substance that Gregor called a mineral as a new element.Klaproth named it “titanium,” which means “Earth” in Latin.용도
As alloy with copper and iron in titanium bronze; as addition to steel to impart great tensile strength; to aluminum to impart resistance to attack by salt solutions and by organic acids; to remove traces of oxygen and nitrogen from incandescent lamps. Surgical aid (fracture fixation).생산 방법
Titanium is the ninth most abundant element and accounts for about 0.63% of the Earth’s crust. Analyses of rock samples from the moon indicate that titanium is far more abundant there; some lunar rocks consist of 12% titanium by weight. World production of titanium sponge metal was estimated at 69,000 metric tons in 1991. The most important titaniumbearing minerals are ilmenite, rutile, and leucoxene. Ilmenite (FeTiO3) is found in beach sands (Australia, India, and Florida) and in rock deposits associated with iron (Norway and Finland). Ilmenite accounts for about 91%of the world’s consumption of titanium minerals and world resources of anatase, ilmenite, and rutile total more than 2 billion tons. Rutile (a form ofTiO2) is less abundant; its chief source is certain Australian beach sands. Two other less prominent forms of TiO2 exist, anatase and brookite. The ores vary around the world in TiO2 content from 39% to 96%. Anatase is used as a food color.정의
A silvery transition metal that occurs in various ores as titanium(IV) oxide and also in combination with iron and oxygen. It is extracted by conversion of titanium(IV) oxide to the chloride, which is reduced to the metal by heating with sodium. Titanium is reactive at high temperatures. It is used in the aerospace industry as it is strong, resistant to corrosion, and has a low density. It forms compounds with oxidation states +4, +3, and +2, the +4 state being the most stable. Symbol: Ti; m.p. 1660°C; b.p. 3287°C; r.d. 4.54 (20°C); p.n. 22; r.a.m. 47.867.일반 설명
TITANIUM is a gray lustrous powder. TITANIUM can be easily ignited and burns with an intense flame. The very finely powdered material may be ignited by sparks.공기와 물의 반응
Highly flammable. Pyrophoric in dust form [Bretherick 1979, p. 104]. Titanium is water-reactive at 700C, releasing hydrogen, which may cause an explosion [Subref: Mellor, 1941, vol. 7, 19].반응 프로필
TITANIUM reacts violently with cupric oxide and lead oxide when heated. When titanium is heated with potassium chlorate, potassium nitrate, or potassium permanganate, an explosion occurs [Mellor 7:20. 1946-47]. The residue from the reaction of titanium with red fuming nitric acid exploded violently when the flask was touched [Allison 1969]. Liquid oxygen gives a detonable mixture when combined with powdered titanium, [Kirchenbaum 1956].위험도
Almost all of titanium’s compounds, as well as the pure metal when in powder form, areextremely flammable and explosive. Titanium metal will ignite in air at 1200°C and willburn in an atmosphere of nitrogen. Titanium fires cannot be extinguished by using water orcarbon dioxide extinguishers. Sand, dirt, or special foams must be used to extinguish burningtitanium.건강위험
Inhalation of metal powder may cause coughing,irritation of the respiratory tract, anddyspnea. Intramuscular administration of titaniumin rats caused tumors in blood. Animalcarcinogenicity is not fully established.Human carcinogenicity is not known.화재위험
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.Safety Profile
Questionable carcinogen with experimental tumorigenic data. Experimental reproductive effects. The dust may ignite spontaneously in air. Flammable when exposed to heat or flame or by chemical reaction. Titanium can burn in an atmosphere of carbon dioxide, nitrogen, or air. Also reacts violently with BrF3, CuO, PbOx (Ni + KClO3), metaloxy salts, halocarbons, halogens, CO2, metal carbonates, Al, water, AgF, O2 , nitryl fluoride, HNO3,O2, KClO3, KNO3 , KMnO4, steam @ 704°, trichloroethylene, trichlorotrifluoroethane. Ordinary extinguishers are often ineffective against titanium fires. Such fires require special extinguishers designed for metal fires. In airtight enclosures, titanium fires can be controlled by the use of argon or helium. Titanium, in the absence of moisture, burns slowly, but evolves much heat. The application of water to burning titanium can cause an explosion. Finely dwided titanium dust and powders, like most metal powders, are potential explosion hazards when exposed to sparks, open flame, or high-heat sources. See also TITANIUM COMPOUNDS, POWDERED METALS, and MAGNESIUM.잠재적 노출
Titanium metal, because of its low weight, high strength, and heat resistance, is used in the aerospace and aircraft industry as tubing, fittings, fire walls; cowlings, skin sections; jet compressors; and it is also used in surgical appliances. It is used, too, as controlwire casings in nuclear reactors, as a protective coating for mixers in the pulp-paper industry and in other situations in which protection against chlorides or acids is required; in vacuum lamp bulbs and X-ray tubes; as an addition to carbon and tungsten in electrodes and lamp filaments; and to the powder in the pyrotechnics industry. It forms alloys with iron, aluminum, tin, and vanadium, of which ferrotitanium is especially important in the steel industry. Other titanium compounds are utilized in smoke screens, as mordants in dyeing; in the manufacture of cemented metal carbides; as thermal insulators; and in heat resistant surface coatings in paints and plastics.환경귀착
Titanium is poorly absorbed by plants and animals and is retained to only a certain extent. High levels of titanium in food products can be detects, however, when soil is contaminated by fly-ash fallout or titanium-containing sewage residues and when titanium dioxide is used as a food whitener. Food, which is considered to be the most important source of exposure to titanium, contributes >99% of the daily intake of the element. There are no relevant tolerable intakes for titanium against which to compare estimated dietary intake. Typical diets may contain approximately 0.3–0.5 mg titanium.Titanium content of soil generally ranges from 0.3 to 6%, high levels of which are found in the vicinity of power plants because of combustion of coal.
Titanium concentrations in the atmosphere are comparatively low. Annual average concentrations in urban air are mostly <0.1 mgm-3 and they are lower still in rural air. Air concentrations up to 0.5 mgm-3 have been reported in urban and industrialized areas.
운송 방법
UN2546 Titanium powder, dry, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material.비 호환성
Powder and dust may ignite spontaneously in air. Violent reactions occur on contact with water, steam, halocarbons, halogens, and aluminum. The dry powder is a strong reducing agent; Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause firesor explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.티타늄 준비 용품 및 원자재
원자재
염화 망가니즈(염화 망간)
(S)-tert-Butyl 6-amino-2-((tert-butoxycarbonyl)amino)hexanoate hydrochloride
2-(5-methyl-2-propan-2-yl-phenoxy)ethanamine
High titanium slag
101
TITANIUM SPONGE 40-60 MESH
Copper shavings
Petroleum coke
Dust settling pocket
염화 제1철
염화제이철무수물
염화알루미늄
Titanium tetrachloride solution
사염화실란
금홍석
바나듐(V) 옥시클로라이드
준비 용품
티타늄 공급 업체
글로벌( 274)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21622 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29855 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17346 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7922 | 58 |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 |
sales@zhuoerchem.com | China | 2904 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24727 | 58 |
| henan kanbei chemical co.,ltd | +undefined-86-1523780-4566 +undefined15237804566 |
henankanbeichemical@163.com | China | 511 | 58 |
티타늄 관련 검색:
플루오르화암모늄 타이론 티타늄 트리염화물 헥사플루오로티탄산 암모늄 타이타늄(IV)에톡사이드 옥소(29H, 31H프탈로사이아닌아토(2-)-N29,N30,N31, N32),(SP-5-12)티타늄 이산화티타늄 이리듐 테트라-n-부톡시티탄 디칼륨 헥사플루오로티타늄산염
Stainless Steel Strip, 4,83 mm x 0,15 mm with degraded edges
alloy
Hexafluorotitanic acid
(R,R)-ETHYLENEBIS-(4,5,6,7-TETRAHYDRO-1-INDENYL)-TITANIUM(IV)-(R)-(1,1'-BINAPHTHYL-2)
RAC-DICHLOROETHYLENEBIS-(4,5,6,7-TETRAHYDRO-1-INDENYL)-TITANIUM(IV)
SODIUM TITANIUM FLUORIDE
SILICON TITANIUM OXIDE
titanium alloy (TiAl6V4)