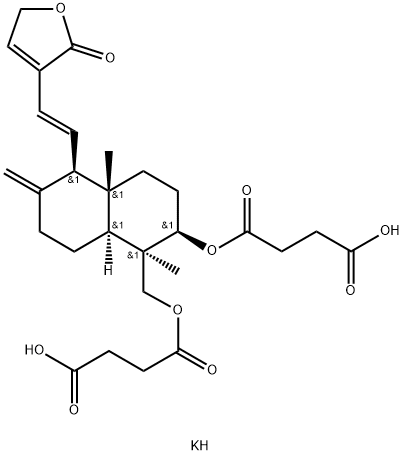
14-데옥시-11,12-디데히드로안드로그라폴라이드3,19-디석시네이트 C화학적 특성, 용도, 생산
개요
This product was included in national standards for chemical drugs.
Injection, freeze-dried powder injection. Used for viral pneumonia and viral
upper respiratory tract infection
물리적 성질
Appearance: white to yellowish crystalline powder, odorless, bitter, slightly wetting. Solubility: slightly soluble in ethanol, insoluble in chloroform, dissolved in 1%
sodium bicarbonate solution.
역사
Andrographolide is unable to satisfy the clinical requirement due to the poor water
solubility. Since the 1970s, researchers had tried to increase the water solubility
through the introduction of different hydrophilic groups in the structure of lactone
and had developed a variety of Andrographis lactone injection. Among them, potassium dehydroandrographolide succinate has the strongest anti-inflammatory and
antipyretic effect. The synthetize of potassium dehydroandrographolide succinate is
as follow described: the formation starts with andrographolide as an intermediate,
after succinic anhydride reaction catalyzed by pyridine, dehydrated andrographolide
succinate half ester was formed then reacted in dilute ethanol solution and potassium bicarbonate to form the final production . Potassium dehydroandrographolide succinate is known as natural antibiotics, and its anti-inflammatory effect is
the best among the 13 andrographolide injections, which has been listed as the top
10 Chinese emergency medicines by the National Chinese Medicine Administration
and has been listed as the first batch of necessary drugs for the National Chinese
Hospital of emergency department .
Indications
This product is contained in the Pharmacopoeia of the People’s Republic of China
(2010).
Injection, freeze-dried powder injection. Used for viral pneumonia and viral
upper respiratory tract infection.
Pharmacology
Potassium dehydroandrographolide succinate and potassium sodium dehydroandrographolide succinate have the same substance of active metabolites in the body
(andrographolide half ester monopotassium salt). As for antipyretic, anti-inflammatory, and antiviral activity, it can promote the anterior pituitary biosynthesis and
release of ACTH and alleviate inflammation through the inhibition of histamineinduced increase in capillary permeability and stimulation to pituitary-adrenal cortical function at the designated site. It can inhibit the binding of DNA with protein in
the process of virus replication, as well as inhibit bacterial endotoxin-induced fever.
It can inactivate adenovirus, influenza virus, respiratory virus, and other viruses and
also inhibit Staphylococcus aureus, Streptococcus aureus, etc. Clinically, it is used
for the treatment of upper respiratory tract infection, bronchial pneumonia, viral
pneumonia, viral enteritis, and hand, foot, and mouth disease .
Clinical Use
Potassium dehydroandrographolide succinate is widely used in the treatment of
viral pneumonia, influenza virus-induced acute upper respiratory tract infection,
acute bronchitis, pediatric bronchial pneumonia, dysentery, epidemic encephalitis,
and asthma attacks, especially for viral and bacterial respiratory tract infections, and
has a significant effect on dysentery, especially for infant pneumonia .
Clinically, potassium sodium dehydroandrographolide succinate is mainly used
for the treatment of upper respiratory tract infection, bronchial pneumonia, viral
pneumonia, viral enteritis, and hand, foot, and mouth disease .
부작용
The adverse reactions of allergic reactions infusion after intravenous drip of
potassium dehydroandrographolide succinate were reported most, some of them
emerge of pimples, abdominal pain, vomiting, dizziness, head swelling, severe
cases, however the sever of them may occur life-threatening anaphylactic shock.
The reactions occurred within 20?min and usually gradually improved in 5–45?min
after symptomatic treatment. There are individuals needing 24? min to start to
recover. In addition, there is also chance to occur thrombocytopenia, liver damage,
blood vessels to stimulate pain, difficulty in breathing, chills, fever, etc. after the use
of potassium dehydroandrographolide succinate .
Skin allergies and diarrhea often occur in children after intravenous drip of
potassium sodium dehydroandrographolide succinate, occasionally allergic shock
and liver damage reports. Serious adverse reactions of potassium sodium dehydroandrographolide succinate injection are systemic damage; the main symptoms
contain allergic shock, allergic reactions, chills, fever, etc., while skin and accessories damage mainly occur rash, of which 53% of patients is children under the age
of 14, 38% of deaths caused by drug-induced allergic shock. According to the 23rd
issue of “adverse drug reactions” published by the National Drug Adverse Drug
Reaction Center, the irrational use of potassium sodium dehydroandrographolide
succinate mainly includes overdose, cross-indications, and allergic constitution .
14-데옥시-11,12-디데히드로안드로그라폴라이드3,19-디석시네이트 준비 용품 및 원자재
원자재
준비 용품