아마폴로네
|
|
아마폴로네 속성
안전
아마폴로네 C화학적 특성, 용도, 생산
Originator
Amafolone hydrochloride,ZYF Pharm ChemicalManufacturing Process
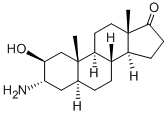
A solution of 70% perchloric acid (60 ml) in water (280 ml) was added to a stirred solution of 5α-androst-2-en-17-one (200.0 g) in ether (1.15 l) at 15°C followed by portionwise addition of N-bromacetamide (112.0 g) over 10 min. After stirring for 1 h the precipitated white crystalline solid was filtered off, washed neutral with ether and water and crystallised to give 2β-hydroxy-3α- bromo-5α-androstan-17-one (172.0 g). The ether layer of the filtrate was washed neutral, dried (Na2SO4) and concentrated to give a second crop (28.0 g). The two crops (200.0 g) were suspended in methanol (1 L), 10 N aqueous potassium hydroxide solution (100 ml) added and the mixture slowly distilled over 45 min. Addition of water precipitated the product as a white solid which was filtered off, washed with water, dried and 88.0 g of 2β,3β-epoxy-5α- androstan-17-one were obtained (crystallisation from ether:light petrol).A solution of the 2β,3β-epoxy-5α-androstan-17-one (30.0 g) in ethanol (130 ml), water (15 ml) and liquid ammonia was heated in an autoclave at 150°C for 6 h and the resultant crystalline suspension evaporated to dryness. Water (35 ml) and acetic acid (36 ml) were added and the solution kept at 90°C for 1 h, cooled and excess water added. The precipitated material was filtered off and the filtrate made alkaline with aqueous 10 N potassium hydroxide solution to precipitate a white solid which was filtered off, washed neutral with water, dissolved in methylene chloride, the solution dried (Na2SO4) concentrated and ether added to give 2β-hydroxy-3α-amino-5α-androstan-17-one (14.3 g), melting point l92°-195°C.
In practice it is usually used as hydrochloride.
Therapeutic Function
Antiarrhythmic아마폴로네 준비 용품 및 원자재
원자재
준비 용품
아마폴로네 공급 업체
글로벌( 5)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | |
support@targetmol.com | United States | 38631 | 58 |