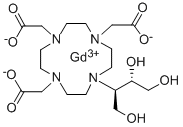
Gadobutrol
|
|
Gadobutrol 속성
- 녹는점
- >264°C (dec.)
- 밀도
- 1.3 g/mL at 37 °C
- 저장 조건
- Refrigerator
- 용해도
- 물(약간 용해됨)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 흰색에서 황백색까지
- 수소이온지수(pH)
- 6.6-8
- InChIKey
- ZPDFIIGFYAHNSK-IWEVFNEWNA-K
- SMILES
- N1(CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1)[C@H](CO)[C@H](O)CO.[Gd+3] |&1:24,27,r|
안전
| 독성 | LD50 i.v. in mice: 23 mmol/kg (Vogler) |
|---|
Gadobutrol C화학적 특성, 용도, 생산
개요
Gadobutrol is a nonionic, paramagnetic contrast agent developed for tissue contrast enhancement in magnetic resonance imaging (MRI). It has a macrocyclic framework and is neutral. It is a water-soluble, highly hydrophilic compound with a partition coefficient between n-butanol and buffer at pH 7.6 of ~ 0.006.상표명
Gadobutrol is marketed by Bayer AG as Gadovist, and by Bayer HealthCare Pharmaceuticals as Gadavist. In India, it is also marketed by Vivere Imaging as Viv-butrol.부작용
Side effects of Gadobutrol are uncommon but may include: headache, nausea, vomiting, feeling unwell (malaise), dizziness, abnormal or unpleasant taste in your mouth, feeling hot, numbness or tingly feeling, itching or rash, skin redness, or injection site reactions (cold feeling, warmth, pain, or burning). Its more serious side effects include: urinating less than usual or not at all; drowsiness, confusion, mood changes, increased thirst, loss of appetite; swelling, weight gain, shortness of breath; seizures (convulsions); breathing problems; pounding heartbeats or fluttering in your chest; or severe pain, burning, or irritation around the IV needle.폐기물 처리
Expired or waste pharmaceuticals shall carefully take into consideration applicable DEA, EPA, and FDA regulations. It is not appropriate to dispose by flushing the pharmaceutical down the toilet or discarding to trash. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.Gadobutrol 준비 용품 및 원자재
원자재
준비 용품
Gadobutrol 공급 업체
글로벌( 201)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | +86-0571-85134551 |
sales@afinechem.com | China | 15416 | 58 |
| HAINAN POLY PHARMCEUTICAL CO. LTD | +86-0571-89385087 +8613616530509 |
ff@hnpoly.com | China | 118 | 58 |
| Zouping mingyuan import & export trading co., ltd | +86-0543-2240078 +8618364991597 |
myzhao793@163.com | China | 992 | 58 |
| Xilinglab Co., Ltd. | +8618381337976 |
bd@xilinglab.com | China | 86 | 58 |
| BOC Sciences | 16314854226; +16314854226 |
inquiry@bocsci.com | United States | 19654 | 58 |
| Guangzhou Tosun Pharmaceutical Ltd | +86-020-61855200-902 +8618124244216 |
info@upharm.cn | China | 897 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569262 +86-15003564040 |
1087@dideu.com | China | 3288 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20232 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 |
sales@sjar-tech.com | China | 485 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21628 | 55 |