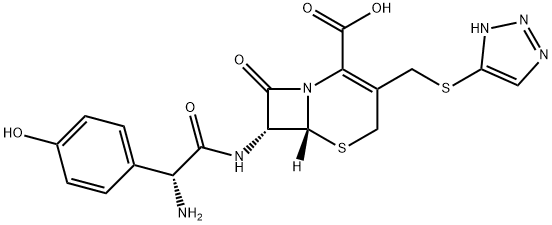
Cefatrizine
|
|
Cefatrizine 속성
- 끓는 점
- 948.1±65.0 °C(Predicted)
- 밀도
- 1.3806 (rough estimate)
- 굴절률
- 1.7000 (estimate)
- 저장 조건
- Store at -20°C
- 용해도
- DMSO: 95 mg/mL (201.02 mM);Ethanol: 23 mg/mL (48.67 mM)
- 산도 계수 (pKa)
- 2?+-.0.50(Predicted)
- 수용성
- Water: 95 mg/mL (201.02 mM)
- CAS 데이터베이스
- 51627-14-6(CAS DataBase Reference)
안전
| 독성 | LD50 in male, female mice, male, female rats (mg/kg): 6880, 6410, 4325, 4325 i.p. (Matsuzaki) |
|---|
Cefatrizine C화학적 특성, 용도, 생산
개요
Cefatrizine was synthesized by BristolMyers Co. and Smith Klein & French Laboratories in 1974. It shows two to four times higher activity against gram-positive and four to eight times higher activity against gram-negative bacteria than cephalexin. Cefatrizine also shows excellent oral absorption, and its in vivo activity is 30 to 500 times higher than that of cephalexin.용도
Cefatrizine is a new orally administered cephalosporin with excellent activity against gram-positive cocci, inhibiting all except enterococci at minimal inhibitory concentrations below 1 μg/ml.정의
ChEBI: A cephalosporin compound having (1H-1,2,3-triazol-4-ylsulfanyl)methyl and [(2R)-2-amino-2-(4-hydroxyphenyl)]acetamido side-groups. An antibacterial drug first prepared in the 1970s, it has more recently been found to be n inhibitor of eukaryotic elongation factor-2 kinase (eEF2K), which is known to regulate apoptosis, autophagy and ER stress in many types of human cancers.Antimicrobial activity
A semisynthetic cephalosporin formulated for oral use. The spectrum is similar to that of cefalexin but it is more active against H. influenzae. Wide strain variations in susceptibility have been reported.It is only partially absorbed when given by mouth. A 500 mg oral dose achieves a concentration of c. 6 mg/L after 1–2 h. The normal half-life of 2.5 h is extended to 5.5 h in end-stage renal failure. Distribution resembles that of cefalexin. It crosses the placenta readily. Plasma protein binding is 40–60%.
Urinary recovery in 6 h is 35% of an oral dose, producing urinary levels of 50–1500 mg/L. It is presumed that the remainder is metabolized, but no metabolites have been identified.
Apart from some mild diarrhea, it is well tolerated. It is available in Japan.
Safety Profile
Moderately toxic byintraperitoneal and subcutaneous routes. An experimentalteratogen. Other experimental reproductive effects. Whenheated to decomposition it emits very highly toxic fumesof NOx and SOx.Cefatrizine 준비 용품 및 원자재
원자재
준비 용품
Cefatrizine 공급 업체
글로벌( 111)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29826 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 19892 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34571 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 9030 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 |
gksales1@gk-bio.com | China | 9322 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 |
info@dycnchem.com | China | 52861 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
Cefatrizine 관련 검색:
세파렉신
Cefatrizine P.G Jp13
CEFATRIZINE PROPYLENE GLYCOL EPC(CRM STANDARD),CEFATRIZINE PROPYLENE GLYCOL EPC(CRM STANDARD)
CEFATRIZINE IMPURITY A
7-TACA for cefatrizine P.G
7-amino-3-(1,2,3-triazole-4-ylthio)methyl cephalosporanic acid Inter.cefatrizine (7-TACA)
7-TACA / for Cefatrizine
Zileuton
Cefatrizine propylene glycolate
Cefatrizine
7-Amino-3-(1,2,3-triazol-4-ylthio)methyl cephalosporanic acid
5-Methylmercapto-1,2,3-triazole
CEFADROXIL
CEFATRIZINE PROPYLENE GLYCOL,CEFATRIZINE PROPYLENE GLYCOL,propyleneglycol-cefatrizin