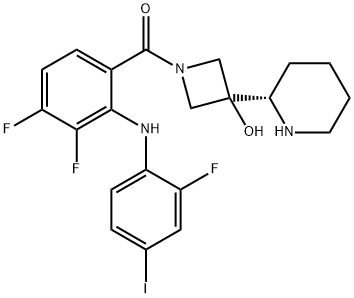
코비메티닙 C화학적 특성, 용도, 생산
개요
Cobimetinib, codeveloped by
Genentech and Exelixis, was approved in August 2015 in
Switzerland and November 2015 in the U.S. and Europe for the
treatment of unresectable or metastatic BRAFV600 mutationpositive
melanoma when used in combination with vemurafenib. Cobimetinib is a potent, highly selective reversible
inhibitor of mitogen-activated protein kinases (MEK) 1 and
2,120 which serves to inhibit phosphorylation of ERK1/2,121
disrupting the MAPK pathway which is responsible for cell
proliferation, cell survival, and migration.122 Combination of
cobimetinib with vemurafenib, an important BRAF inhibitor,123
enables targeting of multiple points on the MAPK pathway,
leading to overall enhanced tumor cell apoptosis and response
as compared to stand-alone treatment with vemurafenib.124
Specifically, in a representative trial of previously untreated
patients with BRAFV600 mutation-positive, unresectable, stage
IIIc or IV melanoma, combination of these two therapies led to
a significantly improved progression-free survival and overall
response rate versus patients treated only with vemurafenib.
용도
A potent, selective, orally bioavailable inhibitor of MEK1, a component of the RAS/RAF/MEK/ERK pathway. It inhibits proliferation and stimulates apoptosis in a variety of human tumor cell lines. In preclinical xenograft models, oral administration of XL518 results in sustained inhibition of pERK in tumor tissue, but not brain tissue, leading to tumor growth inhibition and regression at well tolerated doses.
상표명
Cotellic
Pharmacokinetics
Cobimetinib has only moderate oral
bioavailability (46%), likely due to metabolism rather
than incomplete absorption. However, it displays
prolonged elimination half-life (44 h), which supports
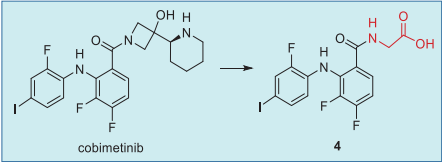
a once-daily dosing regimen (60 mg). Following oral administration, the unchanged
cobimetinib and metabolite 4 were the major
circulating components in the plasma up to 48 hours
post dose (AUC0–48), accounting for 21% and 18% of
all the circulating drug-related components,
respectively (Fig. 4).
target
Primary target: MEK1/2
코비메티닙 준비 용품 및 원자재
원자재
준비 용품