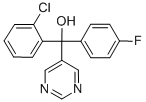
NUARIMOL
|
|
NUARIMOL 속성
- 녹는점
- 126°C
- 끓는 점
- 475.2±40.0 °C(Predicted)
- 밀도
- 1.3255 (estimate)
- 증기압
- 1 x 10-5 Pa at 23 °C
- 저장 조건
- 0-6°C
- 용해도
- 클로로포름(약간 용해됨), 메탄올(약간 용해됨)
- 산도 계수 (pKa)
- 11.37±0.29(Predicted)
- 수용성
- 25°C에서 26mg l-1(pH 7)
- 색상
- 흰색에서 연한 노란색
- BRN
- 6223667
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 그림문자(GHS): |

|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | |||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||
| 예방조치문구: |
|
NUARIMOL C화학적 특성, 용도, 생산
용도
Nuarimol is a systemic fungicide with both curative and protective activity. It controls a wide range of pathogenic fungi, e.g. Cercosporella spp., Septoria spp., Ustilago spp., powdery mildews, leaf spot, etc. in cereals (as a foliar spray and as a seed treatment), powdery mildews on pome fruits, stone fruits, vines, cucurbits and other crops and scab on apples.신진 대사 경로
Photolytic degradation is the primary dissipation mechanism of nuarimol in the environment. Major degradation reactions observed on plants/soil surfaces and water include hydroxylation of the phenyl groups, oxidation of the carbinol carbon atom, dehalogenation and carbinol dehydroxylation (Scheme 1). The primary metabolic pathway of nuarimol in rats involves mainly aryl hydroxylation (Scheme 2).Degradation
Nuarimol is stable to hydrolytic degradation when maintained in sterile buffered solutions (pH 3,6 and 9) in the dark at 52 °C (Saunders, 1977). It was readily degraded in distilled water via photolysis. The photolytic DT50 of nuarimol was approximately 1 hour. Numerous photoproducts were observed; however, no structural characterisation information was reported (Zornes and Donoho, 1978).Nuarimol was extensively photodegraded on solid surfaces. More than 80 photodegradation products were observed when nuarimol was exposed to sunlight on a stainless steel surface for up to 150 hours (Althaus, 1980a). All photoproducts were formed at very low levels (less than 3% each). The structures of 23 photoproducts were identified. An abbreviated photodegradation pathway of nuarimol (based on products accounting for 1% or greater) is presented in Scheme 1. These products were generated from the following reactions: aryl hydroxylation of the chlorophenyl and fluorophenyl moieties (to yield 2, 3), cleavage of the pyrimidine ring and the oxidation of the carbinol carbon atom (4,5) and dehydroxylation of the carbinol moiety (6). Carboxylic acid fragments from the phenyl(7,8,9) and pyrimidine moieties (10), resulting from the cleavage of the parent phenyl and pyrimidine linkages, were also observed.
NUARIMOL 준비 용품 및 원자재
원자재
준비 용품
NUARIMOL 공급 업체
글로벌( 64)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49732 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29808 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 |
info@dycnchem.com | China | 52849 | 58 |
| Henan Alfa Chemical Co., Ltd | +8615838112936 |
alfa10@alfachem.cn | China | 13194 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 49984 | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8618501500038 |
3899766280@qq.com | China | 26362 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39927 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131957 | 58 |