2-(Trimethylsilyl)ethanol
|
|
2-(Trimethylsilyl)ethanol 속성
- 끓는 점
- 71-73 °C35 mm Hg(lit.)
- 밀도
- 0.825 g/mL at 25 °C(lit.)
- 굴절률
- n
20/D 1.423(lit.)
- 인화점
- 123 °F
- 저장 조건
- Sealed in dry,2-8°C
- 용해도
- 클로로포름(약간 용해됨), 에틸아세테이트(약간 용해됨)
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- 15.38±0.10(Predicted)
- 색상
- 무색의
- Specific Gravity
- 0.825
- 수용성
- 녹는
- Hydrolytic Sensitivity
- 4: no reaction with water under neutral conditions
- BRN
- 1732034
- 안정성
- 흡습성
- InChIKey
- ZNGINKJHQQQORD-UHFFFAOYSA-N
- CAS 데이터베이스
- 2916-68-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-36/37/38 | ||
| 안전지침서 | 16-37/39-26-24/25 | ||
| 유엔번호(UN No.) | UN 1987 3/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | KM5480000 | ||
| 위험 참고 사항 | Flammable | ||
| TSCA | No | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29310095 | ||
| 독성 | mouse,LD50,intraperitoneal,1122mg/kg (1122mg/kg),Doklady Akademii Nauk SSSR. Proceedings of the Academy of Sciences of the USSR. For English translation, see DBIOAM and DKBSAS. Vol. 229, Pg. 1011, 1976. |
2-(Trimethylsilyl)ethanol C화학적 특성, 용도, 생산
화학적 성질
CLEAR COLOURLESS LIQUID물리적 성질
bp 50–52 °C/10 mmHg, 71–73 °C/35 mmHg; d 0.825 g cm?3.용도
2-(Trimethylsilyl)ethanol is a protecting reagent for carboxyl, phosphoryl, hydroxyl, and amino groups. It participates in the reactions of Phenol and Acid Protection, Alcohol Protection, Hemiacetal Protection, Amine Protection, Enol Ether Synthesis, Carbohydrate Chemistry etc.제조 방법
Three methods of preparation have been reported: (a) from the treatment of ethyl bromoacetate with zinc followed by the reaction with chlorotrimethylsilane1 and subsequent reduction of the resultant ethyl trimethylsilylacetate with lithium aluminum hydride2,3 or borane¨Ctetrahydrofuran(eq 1); (b) from the hydroboration/oxidation or oxymercuration/demercuration of vinyltrimethylsilane (eq 2); and (c)most conveniently, by the reaction of the Grignard reagent formed from (chloromethyl)trimethylsilane with paraformaldehyde (eq 3).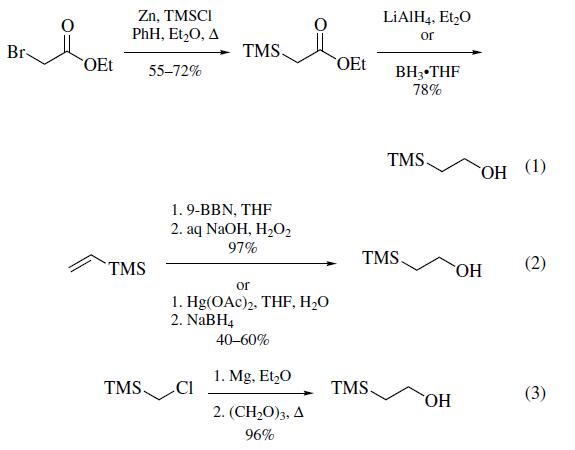
Purification Methods
If the NMR spectrum is not clean, then dissolve the alcohol in Et2O, wash it with aqueous NH4Cl solution, dry (Na2SO4), evaporate and distil it. The 3,4-dinitrobenzoyl derivative has m 66o (from EtOH). [NMR: Speier et al. J Am Chem Soc 79 974 1957, Z Naturforsch 14b 137 1959, Beilstein 4 IV 3951.]2-(Trimethylsilyl)ethanol 준비 용품 및 원자재
원자재
준비 용품
2-(Trimethylsilyl)ethanol 공급 업체
글로벌( 376)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5858 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 |
sales@frappschem.com | China | 880 | 50 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18644 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21639 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 |
FandaChem@Gmail.com | China | 9220 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32820 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29885 | 58 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 |
figo.gao@foxmail.com | China | 8497 | 58 |
2-(Trimethylsilyl)ethanol 관련 검색:
트리메틸술폭소늄 클로라이드 염화트리메틸벤질암모늄 2-메톡시 에탄올 에틸알코올 트리메톡시비닐실란 초산에틸 2- (트리메틸 실릴)에 톡시 메틸 클로라이드
N-(Trimethylsilyl)acetamide
allyltrimethylammonium chloride
Silicone rubber,methyl-vinyl
TERT-BUTYL TRIMETHYLSILYLACETATE
FMOC-TYR(PO3(MDPSE)2)-OH
(TRIMETHYLSILYL)ACETIC ACID
2-(METHYLDIPHENYLSILYL)ETHANOL
3-(TRIMETHYLSILYL)-1,2-PROPANEDIOL
Ethyl (trimethylsilyl)acetate
METHYL (TRIMETHYLSILYL)ACETATE
2-(TRIMETHYLSILYL)ETHOXYMETHYLTRIPHENYLPHOSPHONIUM CHLORIDE







