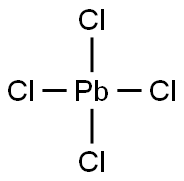납(IV)염화물.
|
|
납(IV)염화물. 속성
- 녹는점
- -15°C
- 끓는 점
- decomposes at ~50℃ [CRC10]
- 물리적 상태
- 노란색 유성 액체
- 색상
- 노란색 유성 액체
안전
납(IV)염화물. C화학적 특성, 용도, 생산
화학적 성질
yellow oily liquid [CRC10]Melting Point: ?15 [CRC10]물리적 성질
Yellow oily liquid; fumes in air; unstable at ordinary temperatures; solidifies at -15°C; decomposes at 50°C.제조 방법
Lead tetrachloride may be prepared by dissolving lead dioxide in cold concentrated hydrochloric acid at 0°C:PbO2 + 4HCl → PbCl4 + 2H2O
However, in the above method some amount of lead dichloride may form, especially if the temperature is above 0°C.
The preferred preparation method is to introduce chlorine into the solution while dissolving lead dioxide in cold concentrated HCl. This prevents decomposition of PbCl4 to PbCl2 and enhances the formation of chloroplumbic acid, H2PbCl6 in solution. Addition of ammonium chloride precipitates out yellow ammonium chloroplumbate, (NH4)2PbCl6 , which is filtered out. The yellow precipitate, on treatment with cold concentrated sulfuric acid, forms lead tetrachloride, which separates out as a yellow oily liquid. The reactions are:
PbO2 + 6HCl → H2PbCl6 + 2H2O
H2PbCl6 + 2NH4Cl → (NH4)2PbCl6 + 2HCl
(NH4)2PbCl6 + H2SO4 → PbCl4 + (NH4)2SO4 + 2HCl
납(IV)염화물. 준비 용품 및 원자재
원자재
준비 용품
납(IV)염화물. 공급 업체
글로벌( 3)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 8722 | 58 |