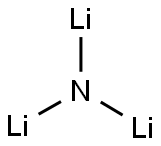
질화리튬 C화학적 특성, 용도, 생산
화학적 성질
reddish brown crystal(s) or freely flowing powder(s); slowly decomposed by atmospheric moisture; ruby red; hexagonal, a=0.3658 nm, c=0.3882nm; conductivity, 227°C, 0.04 (ohm· cm)?1; one of most effective solid ionic conductors; can be prepared by direct reaction of Li and nitrogen; used as a nitriding agent in metallurgy [HAW93] [STR93] [CIC73] [KIR81]
용도
Lithium nitride is used in metallurgy and chemical synthesis. It is also used to store hydrogen and acts as a source of the nitride ion. It is involved in the preparation of lithium hydride and lithium amide as well as a reducing agent.
제조 방법
Lithium nitride is prepared by the reaction of nitrogen gas with lithium metal. The
reaction may be carried out at temperatures well above the melting point of lithium metal or using solid lithium metal at temperatures even below 100°C. Lithium nitride, a
red crystalline solid, reacts with water to yield lithium hydroxide and ammonia. It is
ultimately converted to lithium carbonate in the air. The compound readily reacts with
water and carbon dioxide. It is also flammable, particularly when it is finely divided. For
these reasons lithium nitride is stored and handled under an inert atmosphere.
일반 설명
A reddish brown powder. Insoluble in most organic solvents. Used in metallurgy and chemical synthesis.
반응 프로필
LITHIUM NITRIDE is a strongly basic reducing agent. Incompatible with oxidizing agents such as atmospheric oxygen. Violently incompatible with acids, particularly oxidizing acids. Reacts violently with copper(I) chloride to produce metallic copper [Mellor, 1940, vol.8, 100]. Reacts exothermically with silicon tetrafluoride, leading to explosion [Chem. Brit., 1979, 15, 282-283].
건강위험
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
화재위험
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Safety Profile
A powerful reducing
agent. Upon contact with moisture, it
decomposes into lithmm hydroxide, lithium
compounds, and ammonia. The powder
may ignite spontaneously in moist air.
Flammable at elevated temperatures; ignites
and burns intensely in air. Violent reaction
with dicon tetrafluoride, copperp) chloride
+ heat. To fight fire, use dry chemical, sand,
graphite; avoid use of water or carbon
tetrachloride. When heated to
decomposition it emits very toxic fumes of
Liz0 and NOx. Used as a strong reducing
agent in organic synthesis and a solid
electrolyte in lithium batteries. See also LITHIUM COMPOUNDS and
NITRIDES.
질화리튬 준비 용품 및 원자재
원자재
준비 용품