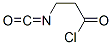3-이소시아나토프로파노일클로라이드
|
|
3-이소시아나토프로파노일클로라이드 속성
안전
3-이소시아나토프로파노일클로라이드 C화학적 특성, 용도, 생산
제조 방법
Diphosgene is toxic. The reaction should be carried out in a well-ventilated hood. A 500-mL, twonecked flask was equipped with a thermometer and a reflux condenser protected at the top by a calcium chloride guard tube. A Teflon-coated magnetic stirring bar, anhydrous dioxane (250 mL), finely pulverized 3-aminopropanoic acid hydrochloride 1342 (b-alanine HCl) (12.6 g, 0.1 mol), and diphosgene (23.8 g, 14.4 mL, 0.12 mol) were placed in the flask in the order specified. The mixture was stirred and heated at 55–60 C°. After ca. 5 h, the solid had completely dissolved to give a clear solution. Heating was discontinued after a total of 7 h and the solvent was removed under reduced pressure. The residual oil was rapidly distilled under reduced pressure and a distillate amounting to 11.2–12.4 g (84–93%) was collected at 75–85 C°/20 mmHg. Redistillation afforded 10.5–11.8 g (79–88%) of 3-isocyanatopropanoyl chloride 1343 as a colorless liquid (bp 92–94 C°/25 mmHg).Comment: Although the reaction can be carried out with an equimolar amount of trichloromethyl chloroformate, a longer time (15–20 h) is required to reach completion, and the yield is somewhat reduced. If a 1.5–2.0-fold excess of trichloromethyl chloro-formate is used, the reaction time is decreased to ca. 5 h and the yield is increased to 90–95%.
Organic acid chlorides have been prepared with diphosgene in the presence of DMF as a catalyst.
3-이소시아나토프로파노일클로라이드 준비 용품 및 원자재
원자재
준비 용품
3-이소시아나토프로파노일클로라이드 공급 업체
글로벌( 1)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Service Chemical Inc. | -- |
sales@chemos-group.com | Germany | 6373 | 71 |