비스페놀-A
|
|
비스페놀-A 속성
- 녹는점
- 158-159 °C(lit.)
- 끓는 점
- 220 °C4 mm Hg(lit.)
- 밀도
- 1.195
- 벌크 밀도
- 600kg/m3
- 증기압
- <1 Pa (25 °C)
- 굴절률
- 1.5542 (estimate)
- 인화점
- 227 °C
- 저장 조건
- Store below +30°C.
- 용해도
- 0.12g/l 불용성
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- 10.29±0.10(Predicted)
- 색상
- 맑은 연한 노란색에서 연한 주황색까지
- 냄새
- 페놀 같은
- 수용성
- 21.5&C에서 <0.1g/100mL
- Merck
- 14,1297
- BRN
- 1107700
- Dielectric constant
- 5.0(20℃)
- InChIKey
- IISBACLAFKSPIT-UHFFFAOYSA-N
- LogP
- 3.4 at 21.5℃
- CAS 데이터베이스
- 80-05-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 37-41-43-62-52 | ||
| 안전지침서 | 26-36/37/39-45-46-39-36/37-61 | ||
| 유엔번호(UN No.) | UN 3077 9 / PGIII | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | SL6300000 | ||
| 자연 발화 온도 | 510 °C | ||
| TSCA | Yes | ||
| HS 번호 | 29072300 | ||
| 유해 물질 데이터 | 80-05-7(Hazardous Substances Data) | ||
| 독성 | LC50 (96 hr) in fathead minnow, rainbow trout: 4600, 3000-3500 mg/l (Staples) | ||
| 기존화학 물질 | KE-23982 | ||
| 유해화학물질 필터링 | 2019-1-934 | ||
| 중점관리물질 필터링 | 별표1-27 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 4,4'-(1-메틸에틸리덴)비스페놀[4,4'-(1-Methylethylidene)bisphenol; Bisphenol A; 80-05-7] 및 이를 0.3% 이상 함유한 혼합물 |
비스페놀-A C화학적 특성, 용도, 생산
물성
강산화제, 강염기, 물, DMSO, 95% ethanol, aceton에 안정하다.개요
Bisphenol A (4,4’-isopropylidenediphenol, BPA)는 아세톤과 2분자의 페놀 축합에 의해 합성되는 단량체(monomer)이다. 식품의 포장용기로 사용되고 있는 epoxy resin 이나 polycarbonate(PC) plastic의 원료 물질로써, 비스페놀A는 캔의 부식을 방지하거나, 열에 대한 저항성과 내구성을 높이기 위하여 플라스틱 병의 첨가물로 사용되고 있다.용도
비스페놀 A(BPA: Bisphenol A)는 지구상에서 가장 흔히 쓰이는 화합물의 하나이며, 폴리카보네이트와 에폭시 수지 등의 생산에 쓰인다.개요
Reports of bisphenol- A sensitization, particularly in workers at epoxy resin plants, are controversial. Bisphenol-A was also reported as an allergen in fiberglass, semisynthetic waxes, footwear and dental materials.화학적 성질
Bisphenol A is a white or tan crystals or flakes with a mild phenolic odor and a very low vapor pressure (ECB, 2003). It is mildly soluble in water. It is not considered to be an explosive in the conventional sense but can pose a hazard as a finely powdered material in air (ECB, 2003). It is not considered to be a chemical oxidizer.역사
Bisphenol A (BPA) was first synthesized in 1891, but it was not used widely until applications in the plastics industry were identified in the 1950s (University of Minnesota, 2008). While the most prominent use of BPA is in the manufacture of polycarbonate plastic and epoxy resins, it is also used in the production and processing of polyvinyl chloride (PVC) and modified polyamide and in the manufacture of carbonless and thermal paper, wood filler, adhesives, printing inks, surface coatings, polyurethane, brake fluid, resin-based dental composites and sealants, flame retardants, paints, and tires (ECB, 2003; EFSA, 2006).
용도
Bisphenol A (BPA) is used as the constitutional monomer or the monomeric building block of polycarbonate plastics, either by trans-esterification with diphenyl carbonate or via the interfacial process with a monohydroxylic phenol. Together with epichlorohydrin, BPA is also used as a major component of epoxy resins. Bisphenol A-polycarbonate plastics are in turn used in the manufacture of plastic food containers such as reusable water bottles, while epoxy resins are used as inner linings of tin cans. In addition, BPA is also used as an additive in other plastics and polymers, particularly as an antioxidant or stabilizer in polyvinyl chloride, printer ink, and in some other products.정의
ChEBI: A bisphenol that is 4,4'-methanediyldiphenol in which the methylene hydrogens are replaced by two methyl groups.제조 방법
The formation of bisphenol A is thought to proceed as follows: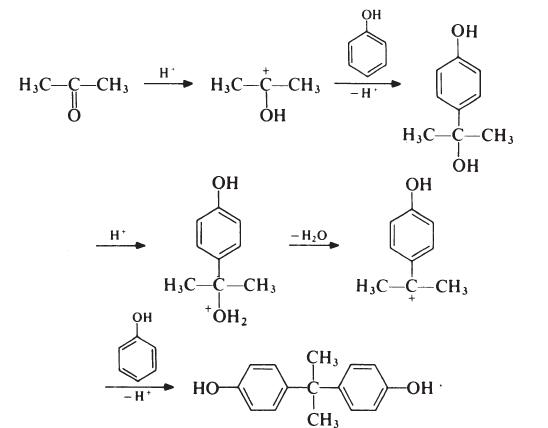
Although the reaction theoretically requires the molar ratio of reactants to be 2: 1, an improved yield of bisphenol A is obtained if additional phenol is present; the optimum molar ratio is 4: 1. In a typical process, the phenol and acetone are mixed and warmed to 50??C. Hydrogen chloride (catalyst) is passed into the mixture for about 8 hours, during which period the temperature is kept below 70??C to suppress the formation of isomeric products. Bisphenol A precipitates and is filtered off and washed with toluene to remove unreacted phenol (which is recovered). The product is then recrystallized from aqueous ethanol. Since epoxy resins are oflow molecular weight and because colour is not normally particularly important, the purity of bisphenol A used in resin production is not critical. Material with a p,p'-isomer content of 95-98% is usually satisfactory; the principal impurities in such material are o,p'- and o,o'-isomers.
일반 설명
White to light brown flakes or powder. Has a weak medicine odor. Sinks in water.공기와 물의 반응
The finely powdered resin is a significant dust explosion hazard. Insoluble in water.반응 프로필
Bisphenol A is incompatible with strong oxidizers. Bisphenol A is also incompatible with strong bases, acid chlorides and acid anhydrides.위험도
Poison; moderately toxic; teratogen; irritant.건강위험
Dusts irritating to upper respiratory passages; may cause sneezing.화재위험
Bisphenol A is combustible. Bisphenol A may form explosive dust clouds. Static electricity can cause its dust to explode.색상 색인 번호
Bisphenol A is used with epichlorhydrin for the synthesis of epoxy resins bisphenol-A type, for unsaturated polyester and polycarbonate resins, and epoxy di(meth)acrylates. In epoxy resins, it leads to bisphenol-A diglycidyl ether, which is the monomer of bisphenol-A-based epoxy resins. Reports of bisphenol-A sensitization are rare and concern workers at epoxy resin plants, after contact with fiber glass, semi-synthetic waxes, footwear, and dental materials. It is also a possible sensitizer in vinyl gloves.잠재적 노출
Workers engaged in the manufacture of epoxy, polysulfone, polycarbonate and certain polyester resins. It is also used in flame retardants, rubber chemicals, and as a fungicide. Bisphenol A (BP A), an environmental estrogen, is found in a wide variety of products, including polycarbonate bottles food and drink containers. According to 2008 research conducted at University of Cincinnati, when it comes to BPA, it’s not whether polycarbonate bottles are new or old but the liquid’s temperature that has the greatest impact on how much BPA is released. When exposed to boiling hot water, BPA was released 55 times more rapidly than exposure to cold water.환경귀착
Bisphenol A can be released into the environment during the production, processing, and use of BPA-containing materials, although levels in environmental samples are generally very low or undetectable (ECB, 2003). This is because BPA has low volatility and a short half-life in the atmosphere, is rapidly biodegraded in water, and is not expected to be stable, mobile, or bioavailable from soils (ECB, 2003; Cousins et al., 2002).Most environmental releases of BPA are during the manufacture of BPA-containing products when residual BPA in wastewater is released from treatment plants into receiving streams (Cousins et al., 2002). BPA's half-life in soil and water is in the order of 4.5 days while in air it is <1 day (Cousins et al., 2002). It has a low bioconcentration factor and is rapidly metabolized in fish, with a half-life of <1 day (Cousins et al., 2002).
운송 방법
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required.Purification Methods
Crystallise bisphenol from acetic acid/water (1:1). It is used for making polycarbonate bottles and leaches out slowly on heating. It is a known “estrogenic chemical” shown to disrupt chemical signaling in the complex network of glands, hormones and cell receptors which make up the endocrine system. It causes low sperm count and damages the ecosystem by the feminisation of fish, reptiles and birds. [cf Chapter 1, p 3, Beilstein 6 IV 6717.]비 호환성
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides and acid anhydrides.비스페놀-A 준비 용품 및 원자재
원자재
Stirring vessel
아세톤
Phenolic epoxy resin
페놀
폴리(비스페놀 A 탄산)
황산
o-[1-(4-히드록시페닐)-1-메틸에틸]페놀
3-Penten-2-one, 4-hydroxy-, (3Z)-
1H-Inden-5-ol, 2,3-dihydro-1-(4-hydroxyphenyl)-1,3,3-trimethyl-
Phenol, 4-(3,4-dihydro-2,4,4-trimethyl-2H-1-benzopyran-2-yl)-
준비 용품
비스페놀-A 공급 업체
글로벌( 790)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 |
abby@chuanghaibio.com | China | 8773 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3889 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5251 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 |
deasea125996@gmail.com | China | 2472 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 997 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20205 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8615713292910 |
Nancy@kangcang.com.cn | China | 341 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 1015 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3692 | 58 |
비스페놀-A 관련 검색:
트리메틸롤프로판 노닐페놀 아세트아미노펜 수소화비스페놀A 페놀레드 에피클로로하이드린-비스페놀 A 수지 2,2-비스(3,5-디브로모-4-(2,3-디브로모프로폭시)페닐)프로판 1,1,1-트리스(4-하이드록시페닐)에탄 비스페놀 A 디메타크릴산염 2,2-비스(4-하이드록시페닐)헥사플루오로프로판 4,4'-사이클로헥실리덴비스페놀 테트라브로모비스페놀 A 4,4""-술폰일비스(2,6-디브로모페놀) 4,4'-설포닐다이페놀 4,4'-메틸렌비스페놀 4,4'-(9-플루오렌일이덴)다이페놀 아크릴산 t-부틸 4,4'-(1-메틸에틸리덴)비스페놀, 중합체,함유 (클로로메틸) 옥시란, 2-프로펜산