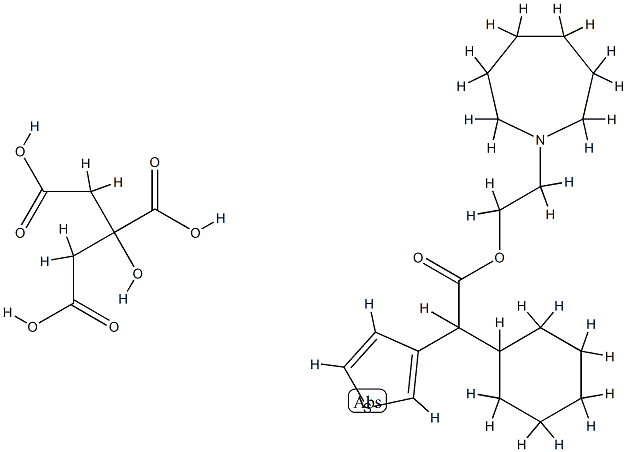
2-(hexahydro-1H-azepin-1-yl)ethyl alpha-cyclohexylthiophene-3-acetate, compound with citric acid (1:1)
|
|
2-(hexahydro-1H-azepin-1-yl)ethyl alpha-cyclohexylthiophene-3-acetate, compound with citric acid (1:1) 속성
- 녹는점
- 115°
안전
2-(hexahydro-1H-azepin-1-yl)ethyl alpha-cyclohexylthiophene-3-acetate, compound with citric acid (1:1) C화학적 특성, 용도, 생산
Originator
Stratene,Innothera,France,1973Manufacturing Process
In a 100 ml flask fitted with a mechanical stirrer, a vertical condensor protected by a calcium chloride stopper, a dropping-funnel and a source of nitrogen were introduced 30 ml of hexamethylenephosphotriamide and 2.3 g (0.1 mol) of finely cut sodium wire. A mixture of 12.3 g (0.1 mol) of (3- thienyl)-acetonitrile and 16.3 g (0.1 mol) of cyclohexyl bromide was then quickly added at a temperature of 20 C. The reaction mixture was then maintained under nitrogen atmosphere and stirred for 12 hours at room temperature. The excess of sodium was destroyed by adding 5 ml of ethanol and the organic solution was slowly poured into 100 ml of a 1 N iced solution of hydrochloric acid. The solution was extracted twice with 100 ml ether. The ethereal phases were collected, washed with water, dried and concentrated under reduced pressure. The crude product was then purified by chromatography on a silica column (150 g of silica) using a 1/1 benzene/cyclohexane mixture as elution agent. The product obtained was rectified by distillation.In this manner, 3.4 g of alpha(3-thienyl)-alpha-cyclohexylacetonitrile were obtained, which represents a yield of 16%.
The nitrile may then be hydrolyzed to cyclohexyl-(3-thienyl)acetic acid which is reacted with 1-(2-chloroethyl)-hexahydro-1H-azepine to give cetiedil. It is commonly used as the citrate.
Therapeutic Function
Vasodilator2-(hexahydro-1H-azepin-1-yl)ethyl alpha-cyclohexylthiophene-3-acetate, compound with citric acid (1:1) 준비 용품 및 원자재
원자재
준비 용품
2-(hexahydro-1H-azepin-1-yl)ethyl alpha-cyclohexylthiophene-3-acetate, compound with citric acid (1:1) 공급 업체
글로벌( 4)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63711 | 58 |
| Shaanxi Dideu Newmaterial Co., Ltd. | 029-63373950 15353716720 |
1052@dideu.com | China | 10011 | 58 |