멜라소폴 C화학적 특성, 용도, 생산
개요
Knowingly or unknowingly, arsenic-containing drugs have been used for treatment of parasitic
conditions for thousands of years. In the late 1800s and early 1900s, Paul Ehrlich introduced the
use of trivalent arsenicals. Melarsoprol, an organoarsenical, came into use in the late 1940s, and
it remains the first-choice drug in the treatment of trypanosomiasis. Until 1990, it also was the only
treatment for late-stage sleeping sickness.
용도
Melarsoprol is a drug used for the treatment of African trypanosomes, a sleeping sickness in humans, a disease that is typically fatal without chemotherapy.
Antimicrobial activity
It is highly and rapidly active against Trypanosoma brucei
gambiense and T. brucei rhodesiense in vitro at submicromolar
concentrations. It is much less active against the trypanosomes
that infect domestic animals, T. congolense and T. vivax.
Co-administration with eflornithine is effective against central
nervous system (CNS) infection with T. brucei in rodent
models, but clinical studies have found the combination less
effective than nifurtimox–eflornithine.
원료
Up to 25% of cases of T. brucei gambiense in Central Africa relapse.
Patients infected with T. brucei rhodesiense normally respond to a
second course of the drug, but those with T. brucei gambiense do
not. In laboratory-generated resistant strains, decreased sensitivity
results from reduced uptake of the drug by bloodstream trypomastigotes
that either lack an adenine/adenosine transporter
(TbAT1) or contain a transporter gene with point mutations.
There is conflicting evidence about the role of this mechanism of
resistance in isolates from patients unresponsive to treatment.
건강위험
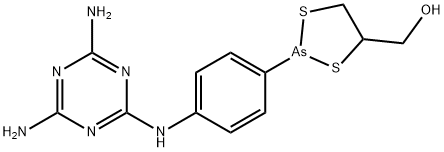
Melarsoprol (4, Mel B, Arsobal C12H15AsN6OS2) is applied for T. b. gambiense or T. b. rhodesiense. The drug, administered intravenously, is a solution containing a combination of BAL (2,3-dimercaptopropanol) and the trivalent arsenic compound, melarsen oxide. Not only can the drug cause serious side effects such as intense dermal irritation, myocarditis, and renal and hepatic damage, but it is also responsible for death in 5% of patients.
Pharmaceutical Applications
Mel B. A derivative of trivalent melarsen oxide and dimercaprol
(BAL), possessing a melaminyl moiety. Formulated
in 3.6% propylene glycol for intravenous administration. It is
almost insoluble in water.
Mechanism of action
It generally is accepted that the enzyme with which melarsoprol reacts is
an enzyme involved in glycolysis, and as a result, inhibition of pyruvate kinase occurs. It is
argued, however, that the inhibition may not occur at pyruvate kinase but, rather, at a step before
the pyruvate kinase. Blockage of glycolysis would be expected to lead to loss of motility and cell
lysis. More recently, Fairlamb et al. have proposed a mechanism of action that results in the
inhibition of trypanothione reductase through the formation of a stable complex between
melaroprol and trypanothione. Melarsoprol
reacts with the cysteine sulfhydryl of trypanothione to form the stable adduct shown in Figure
39.10. Supportive of this mechanism is the synergistic action of melarsoprol with eflornithine
(DMFO). Two drugs that produce sequential blockage of the synthesis of trypanothione.
Pharmacokinetics
Serum levels of 2–4 mg/L were achieved 24 h after administration
of 3.6 mg/kg, falling to 0.1 mg/L at 120 h after
the fourth daily injection. Elimination was biphasic with a
half-life of 35 h. The volume of distribution was 100 L. It
is rapidly metabolized by microsomal enzymes to melarsen
oxide, reaching maximum plasma concentration by 15
min and eliminated with a half-life of 3.9 h. This metabolite
can cross the blood–brain barrier and effect a CNS cure in
mice. Levels of melarsoprol in the cerebrospinal fluid (CSF)
reached around 300 μg/L, about 50 times lower than serum
levels.
Clinical Use
2-p-(4,6-Diamino-s-triazin-2-yl-amino)phenyl-4-hydroxymethyl-1,3,2-dithiarsoline (Mel B, Arsobal) is prepared byreduction of a corresponding pentavalent arsanilate to thetrivalent arsenoxide followed by reaction of the latter with2,3-dimercapto-1-propanol (British anti-Lewisite [BAL]). Ithas become the drug of choice for the treatment of thelater stages of both forms of African trypanosomiasis.Melarsoprol has the advantage of excellent penetration intothe CNS and, therefore, is effective against meningoencephaliticforms of T. gambiense and T. rhodesiense.Trivalent arsenicals tend to be more toxic to the host (as wellas the parasites) than the corresponding pentavalent compounds.The bonding of arsenic with sulfur atoms tends toreduce host toxicity, increase chemical stability (to oxidation),and improve distribution of the compound to the arsenoxide.Melarsoprol shares the toxic properties of other arsenicals, however, so its use must be monitored for signsof arsenic toxicity.
부작용
The propylene glycol formulation can cause tissue trauma
and long-term damage to veins. Drug-induced reactions
include fever on first administration, abdominal colic pain,
dermatitis and arthralgia. Polyneuropathy has been reported
in about 10% of patients. Reactive arsenical encephalopathy
is a serious side effect that occurs in around 10% of
those treated, with death in 1–3% of cases. The frequency of
encephalopathy increases with a rise in the white cell count
or the presence of trypanosomes in the CSF. The causes of
the immunological responses involved in the encephalopathy
and the possible existence of two forms (reactive and hemorrhagic)
are not completely resolved. Studies to identify antiinflammatory
approaches to reduce reactive encephalopathy
in late-stage T. brucei gambiense infection have produced limited
results.
신진 대사
Melarsoprol is administered IV in multiple doses and multiple sessions. Its major metabolite in
humans is the lipophilic melarsen oxide, which can penetrate into the CNS. This metabolite
apparently is responsible for the protein-binding characteristic for melarsoprol.
멜라소폴 준비 용품 및 원자재
원자재
준비 용품