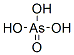Arsenic acid
|
|
Arsenic acid 속성
- 수소이온지수(pH)
- 3.08(1 mM solution);2.31(10 mM solution);1.7(100 mM solution)
안전
| 유엔번호(UN No.) | UN 1553 6.1/ PGI | ||
|---|---|---|---|
| 유엔번호(UN No.) | UN 1554 6.1/ PGII | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | I | ||
| 포장분류 | II | ||
| 독성 | chicken,LDLo,oral,125mg/kg (125mg/kg),"Ueber die Wirkung Verschiedener Gifte Auf Vogel, Dissertation," Forchheimer, L., Pharmakologischen Institut der Universitat Wurzburg, Fed. Rep. Ger., 1931Vol. -, Pg. -, 1931. |
Arsenic acid C화학적 특성, 용도, 생산
개요
Arsenic acid has the molecular formula H3AsO4. More descriptively written as AsO(OH)3, this colorless acid is the arsenic analog of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but only found in solution where it is largely ionized. Its hemihydrate form (H3AsO4·1/2H2O) does form stable crystals. Crystalline samples dehydrate with condensation at 100 °C.Arsenic acid is a tetrahedral species of idealized symmetry C3v with As—O bonds lengths ranging from 1.66 to 1.71? . Being a triprotic acid, its acidity is described by three equilibria:
H3AsO4 ? H2AsO4- + H+ (pk1 =10-2.19)
H2AsO4 ? 5HasO4 2- + H+ (pk2 = 10-6.94)
HasO4 2 ? 5AsO4 3- + H+ (pk3 = 10-11.5)
제조 방법
Arsenic acid is prepared by treating arsenic trioxide with concentrated nitric acid or by combination of arsenic acid with water. The latter reaction is very slow. It is also formed when metaarsenic or pyroarsenic acid is treated with cold water.Arsenic acid 준비 용품 및 원자재
원자재
준비 용품
Arsenic acid 공급 업체
글로벌( 0)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|