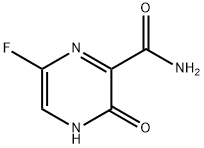
피라진카르복스아미드,6-플루오로-3,4-디히드로-3-옥소-(9CI)
|
|
피라진카르복스아미드,6-플루오로-3,4-디히드로-3-옥소-(9CI) 속성
- 녹는점
- >151°C (dec.)
- 밀도
- 1.78±0.1 g/cm3(Predicted)
- 저장 조건
- under inert gas (nitrogen or Argon) at 2-8°C
- 용해도
- DMSO(30mg/mL), 물(가온 시 12mg/mL)에 용해됨
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- 8.77±0.60(Predicted)
- 색상
- 회백색
- 안정성
- 제공된 대로 구매일로부터 1년 동안 안정적입니다. DMSO 또는 물에 용해된 용액은 -20°에서 최대 3개월 동안 보관할 수 있습니다.
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
피라진카르복스아미드,6-플루오로-3,4-디히드로-3-옥소-(9CI) C화학적 특성, 용도, 생산
개요
Favipiravir, 6-fluoro-3-hydroxypyrazine-2-carboxamide, is a new broad-spectrum antiviral drug targeting RNA-dependent RNA polymerase (RdRp) developed by Japan's Toyama Chemical Pharmaceutical Company. It was approved for marketing in Japan in March 2014 for the treatment of new and recurrent influenza. During the outbreak of the new coronavirus, the results of the Phase I clinical study of the drug published in March 2020 showed that the drug may have the effect of speeding up virus clearance to alleviate the progress of new coronavirus pneumonia.역사
Favipiravir was originally developed in the late 1990s by a company that was later purchased by the Japanese firm Fujifilm as part of its transition from the photo business to healthcare. After being tested against a range of viruses, the drug was approved in Japan in 2014 for emergency use against flu epidemics or to treat new strains of influenza.용도
Favipiravir is used for the treatment of advanced Ebola virus infection in a small animal model. It suppressed the replication of Zaire Ebola virus and prevented a lethal outcome in 100% of the animals. Based on the studies, Favipiravir can be a candidate for the treatment of Ebola hemorrhagic fever. Favipiravir can be used for antiviral treatment of influenza A and B. Studies have shown that in addition to influenza virus, the drug also exhibits good antiviral activity against a variety of RNA viruses, such as Ebola virus, arena virus, Bunia virus, rabies virus and now COVID-19.정의
ChEBI: Favipiravir is a member of the class of pyrazines that is pyrazine substituted by aminocarbonyl, hydroxy and fluoro groups at positions 2, 3 and 6, respectively. It is an anti-viral agent that inhibits RNA-dependent RNA polymerase of several RNA viruses and is approved for the treatment of influenza in Japan. It has a role as an antiviral drug, an anticoronaviral agent and an EC 2.7.7.48 (RNA-directed RNA polymerase) inhibitor. It is a primary carboxamide, a hydroxypyrazine and an organofluorine compound.Mechanism of action
Favipiravir is an antiviral drug that selectively inhibited the RdRP of influenza virus. It showed specific activity against all three influenza A, B, and C (Furuta et al., 2013). It also inhibited the RV replication in HeLa cells, with an EC50 of 29 μg/mL (Furuta et al., 2002). Analysis showed that the primary mechanism of action of favipiravir against the influenza virus was specific inhibition of vRNA polymerase (Furuta et al., 2005). It is predicted that a similar mechanism might occur with other viruses, such as PV and RV, inhibited by favipiravir, which may account for its broad-spectrum inhibition. Mechanistic studies show that the favipiravir and its form favipiravir-RMP (favipiravir-ribofuranosyl-50-monophosphate) do not inhibit influenza RNA polymerase activity, but it is the phosphoribosylated form, favipiravir-ribofuranosyl-50-triphosphate (RTP) that inhibits the enzyme.Synthesis
Favipiravir was synthesized in only six steps from 3-aminopyrazine-2-carboxylic acid with an overall yield of about 22.3%. Key intermediates 3 and 6 were obtained in excellent purity via recrystallization from optimized solvents, which was beneficial to large-scale production. In the key synthetic reaction, 3,6-dichloropyrazine-2-carbonitrile (6) was reacted sequentially, in one pot, with KF and 30% H2O2 to give (after crystallization from 95% EtOH) favipiravir as colorless crystals, with a 60% yield for this final step of the synthesis. (DOI:10.1007/s11696-017-0208-6)
645 grams of sodium hydroxide were dissolved in 9L of water, the temperature was lowered to 5??C, and 1.29 kg of 6-fluoro-3-hydroxy-2-cyanopyrazine was added in batches, stirred, and heated slightly, and the temperature of the reaction system was controlled to -10??C, it takes 3.5 hours to complete the addition, after holding for 1 hour, the temperature is raised to 40??C for 1 hour. Add 100g of activated carbon to the reaction solution, hot filter, cool the mother liquor to 5??C, adjust the pH to 3-4 with concentrated hydrochloric acid, precipitate a large amount of solids, filter and dry to obtain a crude off-white powder, beaten with 2.8 liters of 15% methanol aqueous solution and filter After drying, 1.34 kg of white powder favipiravir was obtained. 1H-NMR (DMSO, 600MHz): |? 13.38 (s, 1H), 8.73 (1s, 1H), 8.51-8.49 (d, J=12, 2H) (yield 91%).
피라진카르복스아미드,6-플루오로-3,4-디히드로-3-옥소-(9CI) 준비 용품 및 원자재
원자재
수산화나트륨
3-Aminopyrazine-2-carboxylic acid
Aminopyrazine
Methyl 3-amino-6-bromopyrazine-2-carboxylate
메틸6-브로모-3-메톡시피라진-2-카르복실레이트
준비 용품
피라진카르복스아미드,6-플루오로-3,4-디히드로-3-옥소-(9CI) 공급 업체
글로벌( 531)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| Jinan Jianfeng Chemical Co., Ltd | 0531-88110457; +8615562555968 |
info@pharmachemm.com | China | 225 | 58 |
| Qiuxian Baitai New Material Co., LTD | +8618330912755 |
sale2@hbyalin.com | China | 1677 | 58 |
| HEBEI Meijinnong Import And Export Trade Co.,LTD | +8617363028569 |
admin@mjn-cn.com | China | 93 | 58 |
| Hebei Ningnan Trade Co. LTD | +86-18034019111 |
admin@hbningnan.com | China | 278 | 58 |
| HangZhou RunYan Pharma Technology Co.,LTD. | +86-88660901 +86-18112526015 |
sales@runyanpharma.com | China | 326 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 |
peter@yan-xi.com | China | 6011 | 58 |
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 |
sales06@tgybio.com | China | 920 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |