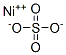황화니켈(흄 및 분진) C화학적 특성, 용도, 생산
개요
Nickel sulphate appears as blue to blue-green transparent crystals and is an odourless
soluble nickel salt. Nickel sulphate is incompatible with strong acids. Nickel sulphate has
extensive industrial applications in nickel patch testing, in nickel plating, as a raw material
for the production of catalysts, in dyeing and printing fabrics as a mordant, and for
blackening zinc and brass and in jewellery manufacture.
화학적 성질
Nickel sulfate is a blue to blue-green crystalline
solid. It has a sweet taste (do not test).
출처
Nickel sulfate heptahydrate occurs in nature as the mineral morenosite.Probably, the most important uses of nickel sulfate are as an electrolyte in nickel plating and electrorefining. Nickel sulfate also is used as a mordant in dyeing and printing textiles. Other uses are in the preparation of many nickel compounds and nickel catalysts; as a reducing agent; for imparting nickel coating or flashing on steel surface; and for blackening zinc and brass.
용도
Nickel sulfate (NiSO4) exists in different states depending on its hydrated forms (where
water molecules bond with ions in suspended substances). Nickel sulfate can be in the form
of greenish-yellow, blue, or green crystals, depending upon the degree of hydration. It is used
in nickel-plating iron and copper, as a catalyst, as a mordant in the textile industry, and as a
coating for other substances.
정의
ChEBI: A metal sulfate having nickel(2+) as the metal ion.
Nickel sulfate, NiS04, is either yellow-green crystals which are soluble in water and insoluble in alcohol and ether,or blue,emerald-green, or green crystals which are soluble in water and alcohol. Derivation is by action of sulfuric acidonnickel. Used in the manufacture of nickel ammonium sulfate, nickel catalysts, nickel plating, and as a mordant in dyeing and printing textiles, coatings,and ceramics.
일반 설명
Anhydrous Nickel sulfate is a yellow-green crystalline solid. Nickel sulfate can also be obtained as a hexahydrate (NiSO4.6H2O (CAS: 10101-97-0) which is blue to emerald green, and as a heptahydrate (NiSO4.7H2O) (CAS: 10101-98-1) , which is green. Samples can contain variable quantities of water, depending on their previous exposure to moisture or conditions. All forms are mildly toxic and are carcinogenic. All are denser than water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used to make other nickel compounds, in printing, and in dyeing of textiles.
공기와 물의 반응
Water soluble yielding acidic corrosive water solutions.
반응 프로필
Gives an acidic solution when dissolved in water. Emits highly toxic fumes of metallic nickel, oxides of sulfur, and oxides of nitrogen when heated to decomposition [Lewis, 3rd ed., 1993, p. 910].
위험도
Toxic material. Questionable carcinogen.
건강위험
Dermatitis.
공업 용도
Nickel sulfate is the most widely used salt for nickel-plating baths, and is known in the plating industry as single nickel salt. It is easily produced by the reaction of sulfuric acid on nickel, and comes in pea-green water-soluble crystalline pellets of the compositionNiSO
4.7H
2O, of a specific gravity of 1.98, melting at about 100 C. Double nickel salt is nickel ammonium sulfate,NiSO
4.(NH
4)
2.SO
4.6H
2O , used especially for plating on zinc. To produce a harder and whiter finish in nickel plating, cobaltous sulfamate, a water-soluble powder of the composition Co(NH
2SO
3)
2.3H
2O, is used with the nickel sulfate. Nickel plate has a normal hardness of Brinell 90 to 140, but by controlled processes file-hard plates can be obtained from sulfate baths. In electroless plating, nickel sulfate, a reducing agent, a pH adjuster, and complexing and stabilizing agents are combined to deposit metallic nickel on an immersed object.The electroless nickel coating is comparable to electrolytic chrome.
Safety Profile
Confirmed human
carcinogen with experimental tumorigenic
data. Poison by intravenous, intraperitoneal,
and subcutaneous routes. Human mutation
data reported. A human skin irritant. When
heated to decomposition it emits very toxic fumes of SOx. See also NICKEL
COMPOUNDS and SULFATES.
잠재적 노출
Nickel sulfate is used in plating baths,
and as an intermediate in the production of nickel ammonium
sulfate; as a mordant in dyeing, and printing textiles;
coatings, and ceramics.
운송 방법
UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard Class: 9; Labels: 9-Miscellaneous
hazardous material, Technical Name Required. UN3288
Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels:
6.1-Poisonous materials, Technical Name Required.
비 호환성
A strong reducing agent. Incompatible
with oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact
may cause fires or explosions. Keep away from
alkaline materials, strong bases, strong acids, oxoacids,
epoxides. The aqueous solution is a weak acid. Sulfates
may react violently with aluminum, magnesium.
폐기물 처리
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal.
황화니켈(흄 및 분진) 준비 용품 및 원자재
원자재
준비 용품