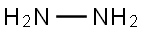히드라진
|
|
히드라진 속성
- 녹는점
- 1,4°C
- 끓는 점
- 65 °C
- 밀도
- 1.011 g/mL at 25 °C
- 증기 밀도
- >1 (vs air)
- 증기압
- 5 mm Hg ( 25 °C)
- 굴절률
- n20/D 1.47(lit.)
- 인화점
- −4 °F
- 저장 조건
- 2-8°C
- 용해도
- very soluble in H2O, ethanol,methanol
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- pK1 (25°): ~6.05
- 색상
- 무색의
- 냄새
- 3~4ppm(평균 = 3.7ppm)에서 비린내 또는 암모니아 같은 냄새가 감지됩니다.
- 폭발한계
- 99.99%
- 수용성
- H2O 및 다음 알코올과 혼합 가능: 메틸, 에틸, 프로필, 이소부틸 [MER06]
- Merck
- 13,4789
- BRN
- 878137
- 노출 한도
- TLV-TWA (skin) 1 ppm (1.3 mg/m3 ) (MSHA and OSHA), 0.1 ppm (ACGIH).
- Dielectric constant
- 52.0(20℃)
- 안정성
- Stability May be an explosion hazard, particularly if heated. Incompatible with sources of ignition, light, shock, strong oxidizing agents, strong acids, metal oxides, nitrous oxide, hydrogen peroxide, most common metals, organic materials, porous materials such as wood, paper, asbestos, soil or rust. Many types of metal may cause rapid d
- LogP
- -0.16 at 20℃
- CAS 데이터베이스
- 302-01-2(CAS DataBase Reference)
- IARC
- 2A (Vol. 4, Sup 7, 71, 115) 2018
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-23/24/25-34-43-50/53-10-51/53-36/37/38-20/21/22-19-11-36/38 | ||
| 안전지침서 | 53-26-36/37-45-61-60-36/37/39-16 | ||
| OEL | Ceiling: 0.03 ppm (0.04 mg/m3) [2-hour] | ||
| 유엔번호(UN No.) | UN 3293 6.1/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | MU7175000 | ||
| F 고인화성물질 | 10-21 | ||
| 자연 발화 온도 | 24 °C on iron rust surface; 270 °C on glass surface | ||
| 위험 등급 | 8 | ||
| 포장분류 | I | ||
| HS 번호 | 28251090 | ||
| 유해 물질 데이터 | 302-01-2(Hazardous Substances Data) | ||
| 독성 | LD50 in mice (mg/kg): 57 i.v.; 59 orally (Witkin) | ||
| IDLA | 50 ppm | ||
| 기존화학 물질 | KE-19981 | ||
| 유해화학물질 필터링 | 97-1-409 | ||
| 중점관리물질 필터링 | 별표1-80 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 히드라진 및 이를 0.1% 이상 함유한 혼합물 |
히드라진 C화학적 특성, 용도, 생산
개요
Hydrazine sulphate, hydrobromide and hydrochloride have been reported to be occupational sensitizers, mainly in soldering flux.화학적 성질
HYDRAZINE, colorless, fuming liquid, decomposes when heated above 350 °C at atmospheric pressure into N2 and NH2, also decomposes in presence of a catalyst (e.g., platinum) into N2 and NH3. Hydrazine burns when ignited in air with a violet-colored flame. The compound is soluble in all proportions with H2O and is soluble in alcohol. Hydrazine forms a hydrate with one molecule of H2O. Upon moderate heating or in a vacuum, the hydrate yields hydrazine and H2O. Hydrazine is a base slightly weaker than NH4OH.물리적 성질
Colorless, mobile, fuming liquid; ammoniacal odor; density 1.0045 g/mL at25°C; refractive index 1.46044 at 22°C; solidifies at 2°C to a white crystallinesolid; boils at 113.5°C; flash point 52°C; burns with a violet flame; vapor pres-sure 14.4 torr at 25°C; critical temperature 379.85°C; critical pressure 145atm; surface tension 66.67 dyne/cm at 25°C; dielectric constant 51.7 at 25°C;viscosity 0.876 centipoise at 25°C; very soluble in water; forms an azeotropewith water at molar composition of 58.5% hydrazine: 41.5% water (71.48%:28.52% by weight), the azeotrope with water boils at 120.5°C; forms hydrazinehydrate at 1:1 molar concentration in water; soluble in alcohols and other polar solvents; pKa 8.1 at 25°C.용도
Hydrazine is used as a high-energy rocket fuel, as a reducing agent, and for preparing organic hydrazine derivatives. The propellant grade of commercial hydrazine is more than 97.7% active. It is also used as an oxygen scavenger in boiler water. Hydrazine has also been used as an experimental drug for treating tuberculosis and sickle cell anemia.생산 방법
Hydrazine is a colorless, fuming, oily liquid with an ammonia-like odor. It should be stored in glass containers in a cool, dark place.Hydrazine is prepared commercially by the Raschig and the urea processes. The Raschig method involves reacting sodium hypochlorite with excess ammonia, flash boiling to recover dilute hydrazine, and fractionating to produce the hydrate. In the urea process, urea is oxidized with hypochlorite to produce the hydrate. Both anhydrous hydrazine and the hydrate are fuming, strongly basic (pKb1=5.52), colorless liquids. Hydrazine may ignite under various circumstances (e.g., on contact with rust) and it decomposes violently in contact with oxidizing materials. It is usually stored under nitrogen to reduce the flammability hazard and to maintain purity.
정의
A colorless liquid that can be prepared by the oxidation of ammonia with sodium chlorate(I) or by the gas phase reaction of ammonia with chlorine. Hydrazine is a weak base, forming salts (e.g. N2H4.HCl) with strong acids. It is a powerful reducing agent, reducing salts of the noble metals to the metal. Anhydrous hydrazine ignites spontaneously in oxygen and reacts violently with oxidizing agents. The aqueous solution, hydrazine hydrate, has been used as a fuel for jet engines and for rockets.일반 설명
Colorless liquid with an ammonia-like odor. A violent poison. Causes delayed eye irritation. Very corrosive, attacks glass, rubber, and cork. Corrodes molybdenum steels such as Allegheny stainless 316.It is a strong reducing agent and a flammable liquid and vapour. Hydrazine is a useful building block in organic synthesis of pharmaceuticals and pesticides. There are many kinds of hydrazine compounds, including hydrazine, 1,1-dimethylhydrazine, and 1,2-dimethylhydrazine. Small amounts of hydrazine occur naturally in plants. Most hydrazines are manufactured for use as rocket propellants and fuels, boiler water treatments, chemical reactants, medicines, and in cancer research. Hydrazines are highly reactive and easily catch fire.
공기와 물의 반응
Fumes in air. Water soluble.반응 프로필
HYDRAZINE are strongly basic and are powerful reducing agents. Note that a 64% solution corresponds to the composition hydrazine hydrate (N2H4.H2O). Spontaneous ignition can occur with hydrogen peroxide and nitric acid. Contact with metallic oxide surfaces may lead to flaming decomposition [Haz. Chem. Data (1966)]. The reaction between 2,4-dinitrochlorobenzene and hydrazine hydrate shattered the reaction flask [Wischmeyer 1967]. Spontaneous ignition occurs when nitrous oxide and hydrazine are mixed [Mellor 8, Supp. 2:214(1967)]. Potassium and sodium dichromate react explosively with hydrazine [Mellor 11:234(1946-1947)]. Hydrazine hydrate reacts with stannous chloride to give stannous dihydrazinechloride, which decomposes explosively when heated [Mellor 7:430(1946-1947)]. Explodes during distillation if traces of air are present. Affected by UV and metal ion catalysis [Merck, 11th ed., 1989].위험도
Severe explosion hazard when exposed to heat or by reaction with oxidizers. Toxic by ingestion, inhalation, and skin absorption; strong irritant to skin and eyes; a confirmed carcinogen.건강위험
Hydrazine is extremely destructive to the tissues of the mucous membranes and upper respiratory tract, eyes, and skin. Skin contact with the liquid can result in severe burns; hydrazine is readily absorbed through the skin, leading to systemic effects, which may include damage to the liver, kidney, nervous system, and red blood cells. Hydrazine vapor is irritating to the nose, throat, and respiratory tract, and inhalation of high concentrations may be fatal as a result of spasm, inflammation, chemical pneumonitis, and pulmonary edema. Symptoms of exposure may include a burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting. Hydrazine vapor is extremely irritating to the eyes and can cause temporary blindness. Eye contact with the liquid can result in severe burns and permanent damage. Hydrazine is not considered to have adequate warning properties. Hydrazine is listed by IARC in Group 2B "possible human carcinogen" and is classified as a "select carcinogen" according to the criteria of the OSHA Laboratory Standard.Chronic exposure to subacute levels of hydrazine can cause lethargy, vomiting, tremors, itching and burning of the eyes and skin, conjunctivitis, and contact dermatitis. Hydrazine has been found to exhibit reproductive and developmental toxicity in animal tests.화재위험
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.인화성 및 폭발성
Hydrazine is a flammable liquid (NFPA rating = 3) over a very broad range of vapor concentrations (4.7 to 100%). Hydrazine may undergo autoxidation and ignite spontaneously when brought in contact with porous substances such as rusty surfaces, earth, wood, or cloth. Fires should be extinguished with water spray, carbon dioxide, or dry chemical extinguishers.Carcinogenicity
Hydrazine and hydrazine sulfate are reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals.환경귀착
Hydrazine can be found in the environment in small quantities and is a component of tobacco smoke. However, hydrazine is primarily an industrial chemical that enters the environment by emissions from its use as an aerospace fuel and from industrial facilities that manufacture, process, and/or use this chemical. Treatment and disposal of wastes containing hydrazine also contribute to environmental concentrations. However, hydrazine rapidly degrades in the environment and is rarely encountered outside the industrial setting.저장
work with hydrazine should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Hydrazine should be used only in areas free of ignition sources. Hydrazine should be stored under nitrogen in containers placed in secondary containers in areas separate from oxidizers and acids.Purification Methods
Hydrazine hydrate is dried by refluxing with an equal weight of KOH pellets for 3hours, then distilled from fresh solid NaOH or BaO in a current of dry N2. Use stainless steel or copper equipment. Hydrazine and its hydrates have VERY IRRITATING and TOXIC vapours and should be used in an efficient fume cupboard. Store in a well-stoppered vessel, preferably under N2. It is a reducing agent. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 469-472 1963.]비 호환성
Hydrazine is a highly reactive reducing agent that forms shock-sensitive, explosive mixtures with many compounds. It explodes on contact with barium oxide, calcium oxide, chromate salts, and many other substances. On contact with metal catalysts (platinum black, Raney nickel, etc.), hydrazine decomposes to ammonia, hydrogen, and nitrogen gases, which may ignite or explode.폐기물 처리
In the event of a spill, remove all ignition sources, soak up the hydrazine with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Evacuation and cleanup using respiratory protection may be necessary in the event of a large spill or release in a confined area. Disposal Excess hydrazine and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. For more information on disposal procedures, see Chapter 7 of this volume.히드라진 준비 용품 및 원자재
원자재
준비 용품
벤조페논히드라존
1-(4-Methoxybutyl)hydrazine
Ethyl carbazate
1-(Tetrahydro-2H-pyran-4-yl)hydrazine
(4-OXO-3,4-DIHYDROPHTHALAZIN-1-YL)ACETIC ACID
1-(4-Morpholinobutyl)hydrazine
2-Hydrazinyl-1-morpholinoethanone
N,N,N',N'-Tetramethylazodicarboxamide
1-메틸-1H-피라졸-4-카르복실산
4-Hydroxy-6-aminopyrimidine
4-HYDRAZINO-6-HYDROXYPYRIMIDINE
(2-MORPHOLIN-4-YL-ETHYL)-히드라진
3-Aminopyrazole-4-carboxylic acid
DESACETYLVINBLASTINEAMIDE
1-(3-(pyrrolidin-1-yl)propyl)hydrazine
3-Hydrazinyl-1-(4-methylpiperazin-1-yl)propan-1-one
(TETRAHYDRO-FURAN-2-YLMETHYL)-HYDRAZINE
5-(2-아미노에틸)-4-메틸티아졸-2-올
Ethyl 3-amino-4-pyrazolecarboxylate
(2-PYRROLIDIN-1-YL-ETHYL)-하이드라진
2-히드라지노-4-(트리플루오로메틸)피리미딘
3-MERCAPTOPICOLINIC ACID
5-사이클로프로필-2H-피라졸-3-일라민
에틸5-아미노-3-(트리플루오로메틸)-1H-피라졸-4-카르복실레이트
4-CHLORO-2-(TRIFLUOROMETHYL)PYRIMIDINE-5-CARBONITRILE
1-(4-(Pyrrolidin-1-yl)butyl)hydrazine
3-Hydrazinyl-1-morpholinopropan-1-one
2-Chloro-4-hydrazinopyrimidine
(3-MORPHOLIN-4-YL-PROPYL)-히드라진
1-(3-Hydrazinylpropyl)pyrrolidin-2-one
2-Hydrazinyl-1-(4-methylpiperazin-1-yl)ethanone
히드라진 공급 업체
글로벌( 126)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 |
abby@chuanghaibio.com | China | 8808 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 |
overseasales1@yongstandards.com | United States | 14332 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49732 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 22855 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; |
peter68@ptchemgroup.com | China | 35425 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 43340 | 58 |
| Aladdin Scientific | |
tp@aladdinsci.com | United States | 57505 | 58 |
| XIAMEN AMITY INDUSTRY AND TRADE CO., LTD. | +8618950047208 |
ellena@amitychem.com | China | 43416 | 58 |
히드라진 관련 검색:
하이드라진, 모노수화물 하이드라진 수화물 4-(트리플루오로메톡시)페닐히드라진 하이드로클로라이드 4-(트리플루오로메틸)페닐히드라진 2-히드라지노-4-(트리플루오로메틸)피리미딘 2-히드라진벤조티아졸
Sumatriptan succinate
Tazobactam Impurity B
Hydralazine
Dihydralazine sulphate
Sumatriptan EP Impurity H
Penehyclidine HCl (Mixture of IsoMers)
4-Hydrazinyl-1-phthalazinaMine
Penehyclidine Impurity 18
3-(2-Aminoethyl)-N-methyl-1H-indole-5-methanesulfonamide
[3-[2-(diMethylaMino)ethyl]-2-[[3-[2-(diMethylaMino)ethyl]-1H-indol-5-yl]Methyl]-1H-indol-5-yl]-N-MethylMethanesulfonaMide, succinate salt
M 8218
N-DesMethyl SuMatriptan