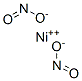
아질산니켈 C화학적 특성, 용도, 생산
일반 설명
A crystalline solid or the solid dissolved in a liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Noncombustible, but accelerates the burning of combustible materials. Used to make other chemicals.
공기와 물의 반응
Slightly soluble in water.
반응 프로필
NICKEL NITRATE is a strong oxidizing agent. A violent explosion occurs if an ammonium salt is melted with a nitrite salt [Von Schwartz 1918. p. 299]. A mixture of potassium cyanide and nitrite salts may cause an explosion [Pieters 1957. p. 30].
건강위험
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
화재위험
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Confirmed carcinogen.
When heated to decomposition it emits
toxic fumes of NOx and Ni.
아질산니켈 준비 용품 및 원자재
원자재
준비 용품