Dasabuvir Sodium C화학적 특성, 용도, 생산
개요
Dasabuvir sodium (Exviera®), an oral non-nucleoside NS5B
polymerase inhibitor discovered and developed by Abbvie, is a
component of an all-oral hepatitis C treatment regimen Viekira
Pak approved by the US FDA in December 2014 for the treatment
of adult patients with chronic genotype 1 (GT1) hepatitis C virus
(HCV) infection. The investigational regimen consists of the
fixed-dose combination of paritaprevir (XXVII) (veruprevir, ABT-
450, vide infra) with ritonavir booster (150/100 mg) co-formulated
with the NS5A inhibitor ombitasvir (XXV) (ABT-267, vide infra) 25 mg, dosed once daily, and nonnucleoside NS5B polymerase
inhibitor dasabuvir (X) (ABT-333) 250 mg with or without ribavirin
(weight-based), dosed twice daily. The drug was granted
breakthrough therapy designation by the US FDA in May 2013.
AbbVie’s application is supported by the data from six Phase III
studies covering over 2300 patients in 25 countries representing
one of the largest clinical programs in hepatitis C research and
development. Across six studies, the 12-week therapeutic regimen
achieved impressive cure rates, notching a 99% sustained virologic
response mark in some populations.
Synthesis
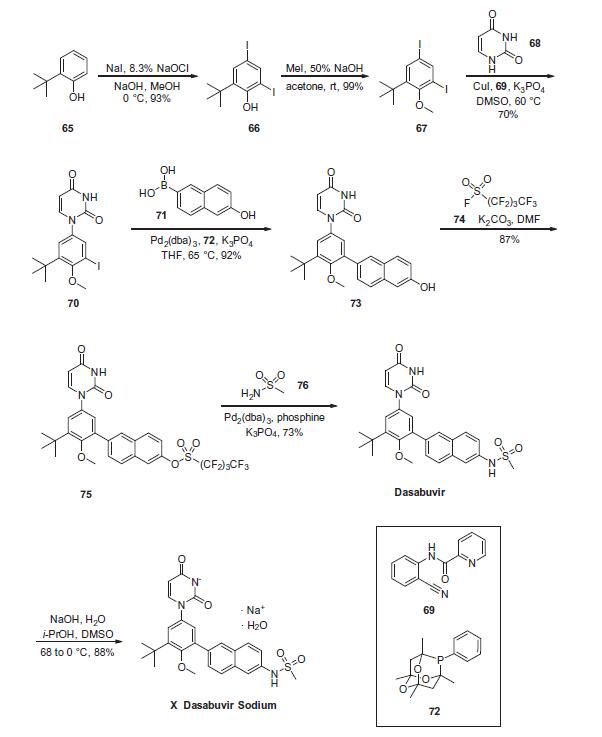
Commercially available 2-tert-butyl phenol (65)
was polyiodinated to furnish diiodophenol 66 in 93% yield. This
was followed by methylation of the phenol to provide methyl phenyl
ether 67 in 99% yield. Next, sequential couplings were
employed to install the periphery about the central phenyl core.
First, Goldberg coupling of 67 with pyrimidine-2,4-(1H,3H)-dione
68 in presence of CuI (10 mol %) and 69 provided compound 70
in 70% yield. Subsequently, the remaining iodide underwent Suzuki
coupling with boronic acid 71 in the presence of Pd2(dba)3 and 72
to yield the naphthol 73 in high yield. Naphthol 73 was then converted
to the corresponding polyfluorinated naphthol sulfonate 75,
which was subsequently converted to dasabuvir through a palladium-
mediated installation of methyl sulfonamide 76. Dasabuvir
sodium (X) was then crystallized upon treatment with aq NaOH
in i-PrOH and DMSO in 88% yield.
Dasabuvir Sodium 준비 용품 및 원자재
원자재
준비 용품







