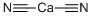CALCIUM CYANIDE suppliers
CALCIUM CYANIDE
- CAS:
- 592-01-8
- MF:
- C2CaN2
- MW:
- 92.11
Properties
- Melting point:
- 640 estimated [KIR78]
- Density
- 1.8 g/cm3
- solubility
- soluble in H2O, ethanol
- form
- white rhombohedral crystals
- color
- white rhomb crystals, crystalline; hygroscopic
- Water Solubility
- soluble H2O, gradually releasing HCN [MER06]
- Exposure limits
- TLV-TWA (measured as CN) skin 5 mg(CN)/ m3 (ACGIH); 5 mg(CN)/m3/10-min ceiling (NIOSH).
Safety Information
- Symbol(GHS)


GHS06,GHS09
- Signal word
- Danger
- Hazard statements
- H300-H410
- Precautionary statements
- P264-P270-P301+P310-P321-P330-P405-P501-P273-P391-P501
- Hazard Codes
- T+,N
- Risk Statements
- 28-32-50/53
- Safety Statements
- 7/8-23-36/37-45-60-61
- RIDADR
- 1575
- HazardClass
- 6.1(a)
- PackingGroup
- I
- HS Code
- 28371990
- Toxicity
- LD50 orally in rats: 39 mg/kg (Smyth)
Use
Calcium cyanide is used mainly for the extraction or cyanidation of gold and silver ores. It is also used in the production of prussiates or ferrocyanides, in the froth flotation of minerals, in processes where gold complexes are adsorbed on carbon, in the manufacture of stainless steel, as a fumigant and rodenticide, and as a cement stabiliser. The main users of cyanides are the steel, electroplating, mining, and chemical industries. The principal cyanide compounds used in industrial operations are potassium and sodium cyanide and calcium cyanide, particularly in metal leaching operations. Cyanides have been well established in uses as insecticides and fumigants; in the extraction of gold and silver ores; in metal cleaning; in the manufacture of synthetic fibres, various plastics, dyes, pigments, and nylon; and as reagents in analytical chemistry. Calcium cyanide decomposes on heating above 350°C, producing toxic fumes including nitrogen oxides and HCN. It reacts violently with water, moist air, carbon dioxide, acids, and acid salts producing highly toxic and flammable HCN. It reacts violently when heated with oxidising substances causing fire and explosion hazard.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "剧毒、有毒、易致癌化学品". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660