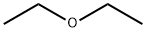Diethyl ether suppliers
Diethyl ether
- CAS:
- 60-29-7
- MF:
- C4H10O
- MW:
- 74.12
Properties
- Melting point:
- -116 °C
- Boiling point:
- 34.6 °C(lit.)
- Density
- 0.714
- vapor density
- 2.6 (vs air)
- vapor pressure
- 28.69 psi ( 55 °C)
- refractive index
- n
20/D 1.3530(lit.)
- Flash point:
- -40 °F
- storage temp.
- Store at RT.
- solubility
- Soluble in water, miscible with ethanol (96 per cent), with methylene chloride and with fatty oils. It is highly flammable.
- form
- Liquid
- Specific Gravity
- 0.714 (20/4℃) ; 0.712 (25℃)
- color
- clearAPHA: ≤10
- Odor
- Pungent odor detectable at 0.33 ppm
- Relative polarity
- 2.9
- explosive limit
- 1.7-36%(V)
- Water Solubility
- 69 g/L (20 ºC)
- FreezingPoint
- -116.3℃
- Merck
- 14,3806
- Henry's Law Constant
- 12.50(x 10-4 atm?m3/mol at 25 °C) (Signer et al., 1969)
- Exposure limits
- TLV-TWA 1200 mg/m3 (400 ppm) (ACGIH and OSHA); STEL 1500 mg/m3 (500 ppm) (ACGIH).
- Dielectric constant
- 4.0(40℃)
- Stability:
- Stable, but light-sensitive, sensitive to air. May contain BHT (2,6-di-tert-butyl-4-methylphenol) as a stabilizer. Substances to be avoided include zinc, halogens, halogen-halogen compounds, nonmetals, nonmetallic oxyhalides, strong oxidizing agents, chromyl chloride, turpentine oils, turps substitutes, nitrates, metallic chlorides. Extre
- LogP
- 0.890
- Surface tension
- 17.153mN/m at 293.15K
- CAS DataBase Reference
- 60-29-7(CAS DataBase Reference)
- NIST Chemistry Reference
- Ethoxy ethane(60-29-7)
- EPA Substance Registry System
- Ethyl ether (60-29-7)
Safety Information
- Symbol(GHS)


GHS02,GHS07
- Signal word
- Danger
- Hazard statements
- H224-H302-H336
- Precautionary statements
- P210-P233-P240-P241-P301+P312-P403+P233
- Hazard Codes
- F+,Xn,T,F
- Risk Statements
- 12-19-22-66-67-39/23/24/25-23/24/25-11
- Safety Statements
- 9-16-29-33-45-36/37-7
- RIDADR
- UN 1155 3/PG 1
- WGK Germany
- 1
- RTECS
- KI5775000
- F
- 10
- Autoignition Temperature
- 160 °C
- TSCA
- Yes
- HS Code
- 2909 11 00
- HazardClass
- 3
- PackingGroup
- I
- Hazardous Substances Data
- 60-29-7(Hazardous Substances Data)
- Toxicity
- LD50 oral (rat) 1215 mg/kg
LC50 inhal (rat) 73,000 ppm (2 h)
PEL (OSHA) 400 ppm
STEL (ACGIH) 500 ppm
- IDLA
- 1,900 ppm [10% LEL]
Use
The acute toxicity of diethyl ether is low. Inhalation of high concentrations can cause sedation, unconsciousness, and respiratory paralysis. These effects are usually reversible upon cessation of exposure. Diethyl ether is mildly irritating to the eyes and skin, but does not generally cause irreversible damage. Repeated contact can cause dryness and cracking of the skin due to removal of skin oils. The liquid is not readily absorbed through the skin, in part because of its high volatility. Diethyl ether is slightly toxic by ingestion. Diethyl ether is regarded as having adequate warning properties. There is no evidence for carcinogenicity of diethyl ether, and no reproductive effects have been reported. Chronic exposure to diethyl ether vapor may lead to loss of appetite, exhaustion, drowsiness, dizziness, and other central nervous system effects.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "易制毒化学品". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660