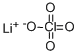Lithium perchlorate suppliers
Lithium perchlorate
- CAS:
- 7791-03-9
- MF:
- ClLiO4
- MW:
- 106.39
Properties
- Melting point:
- 236 °C (lit.)
- Boiling point:
- 430°C
- Density
- 1.13 g/mL at 20 °C
- Flash point:
- 400°C
- solubility
- H2O: 1 M at 20 °C, clear, colorless
- form
- powder
- Specific Gravity
- 2.43
- color
- White
- PH Range
- 6.0 - 7.5
- PH
- 6.0-7.5 (25℃, 5%)
- Water Solubility
- 600 g/L (25 ºC)
- Sensitive
- Hygroscopic
- Merck
- 14,5539
- Stability:
- Strong oxidizer - contact with combustible material may cause fire. Incompatible with organic materials, combustible materials, strong reducing agents.
- CAS DataBase Reference
- 7791-03-9(CAS DataBase Reference)
- NIST Chemistry Reference
- Lithium perchlorate(7791-03-9)
- EPA Substance Registry System
- Perchloric acid, lithium salt (7791-03-9)
Safety Information
- Symbol(GHS)



GHS03,GHS05,GHS07
- Signal word
- Danger
- Hazard statements
- H272-H302-H314-H335
- Precautionary statements
- P210-P260-P280-P301+P312-P303+P361+P353-P305+P351+P338
- Hazard Codes
- O,Xi,Xn,F
- Risk Statements
- 8-36/37/38-9-20/22-11-67
- Safety Statements
- 17-26-36-37/39-36/37/39-16
- RIDADR
- UN 1993 3/PG 1
- WGK Germany
- 1
- F
- 3
- TSCA
- Yes
- HazardClass
- 5.1
- PackingGroup
- II
- HS Code
- 28299000
Use
Lithium perchlorate (LiClO4) is sufficiently soluble (beyond 1Min organic solvents, e.g., EC/DMC) and forms electrolyte solutions with good conductivity (about 9 mS·cm?1 in EC/DMC at ambient temperature). In organic solvents LiClO4 forms thicker solid electrolyte interface (SEI) layers than LiPF6 or LiBF4, but they are less resistive. This fact is attributed to the highly resistive LiF on the surface which is formed by hydrogen fluoride (HF) generated by hydrolysis of fluorine-containing anions, for example, LiBF4 and LiPF6, with traces of moisture and the existing SEI layer [62, 63]. Furthermore, it has a high anodic stability of up to 5.1 V on LiMn2O4 in EC/DMC and is less hygroscopic than LiPF6. Despite its many advantages, the high oxidation state of chlorine (VII) in ClO4 ? results in problems. LiClO4 solutions are thermally unstable and show explosion risks, especially in ethers.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "易制爆、炸药、化学武器等". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660