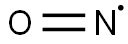NITRIC OXIDE suppliers
NITRIC OXIDE
- CAS:
- 10102-43-9
- MF:
- NO*
- MW:
- 30.01
Suppliers by country/region
Company Type
Properties
- Melting point:
- −163.6 °C(lit.)
- Boiling point:
- −151.7 °C(lit.)
- Density
- d-150.2 (liq) 1.27; Relative d (gas) 1.036 (air = 1); Absolute d (gas) 1.227 (air = 1)
- vapor density
- 1.05 (vs air)
- refractive index
- nD25 1.0002697
- solubility
- At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 21 volumes of water.
- form
- colorless gas
- color
- colorless
- Water Solubility
- slightly soluble H2O [HAW93]
- Merck
- 13,6611
- Exposure limits
- TLV-TWA 25 ppm (~30 mg/m3) (ACGIH, MSHA, and OSHA).
- Stability:
- Spontaneously reacts with oxygen in air to yield brown nitrogen dioxide. Reacts violently or explosively with ammonia and many organic materials.
- InChIKey
- MWUXSHHQAYIFBG-UHFFFAOYSA-N
- CAS DataBase Reference
- 10102-43-9(CAS DataBase Reference)
- EPA Substance Registry System
- Nitric oxide (10102-43-9)
Safety Information
- Symbol(GHS)




GHS03,GHS04,GHS05,GHS06
- Signal word
- Danger
- Hazard statements
- H270-H280-H314-H330
- Precautionary statements
- P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P410+P403
- Hazard Codes
- O,T
- Risk Statements
- 8-23-34-44
- Safety Statements
- 17-23-36/37/39-45
- RIDADR
- UN 1660 2.3
- OEB
- A
- OEL
- TWA: 25 ppm (30 mg/m3)
- WGK Germany
- 1
- RTECS
- QX0525000
- DOT Classification
- 2.3, Hazard Zone A (Gas poisonous by inhalation)
- HazardClass
- 2.3
- Hazardous Substances Data
- 10102-43-9(Hazardous Substances Data)
- Toxicity
- LCLo inhalation in dog: 5000ppm/25M
- IDLA
- 100 ppm
Use
Nitric oxide was prepared in 1772 by Joseph Priestley (1733–1804) and described in his volumes Experiments and Observations of Different Kinds of Air published between 1774 and 1786. Priestley called nitric oxide nitrous air, nitrogen dioxide nitrous acid vapor, and nitrous oxide phlogisticated nitrous air, but also referred to the latter as diminished nitrous air. He observed the change of clear nitric oxide to red nitrogen dioxide. Priestley prepared nitric oxide by reacting nitric acid with a metal such as copper: 3Cu(s) + 8HNO3(aq) → 2NO(g) + 3Cu(NO3)2(aq) + 4H2O(l).
52 supplier list of "NITRIC OXIDE"

- Product Name:Nitric oxide
- Products Intro:Purity: 98% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
- Company Type: Reagent
- Country/Region: INDIA

-
17L$6932.31
- Product Name:NITRIC OXIDE
- Products Intro:Brand:Acccorporation | OriginalID:ING0015891 | Purity:0.00%
- Company Type: Trader
- Country/Region: UNITED STATES

-
1Kit$479.00
- Product Name:Nitric Oxide
- Products Intro:Brand:Usbio | OriginalID:405810
- Company Type: Trader
- Country/Region: UNITED STATES
You can find NITRIC OXIDE suppliers,
manufacturers, and distributors from countries such as United States, CHINA and the India here.
Browse to the tetrahydrofuran product information displayed by the supplier,
as well as the supplier's contact and capability information.