背景及概述[1]
与活泼的环氧化合物相比,二茂铁为金属有机配合物,无论是在酸性还是碱性环境中都具有很好的稳定性。自从 1951 年二茂铁被首次发现以来,研究者们已合成了一系列含环氧结构的二茂铁衍生物如(S)-1-(二苯基磷酸)-2-[(S)-4-异丙基恶唑啉-2-基]二茂铁,这一领域的研究受到国内外学者的关注。

制备[1]
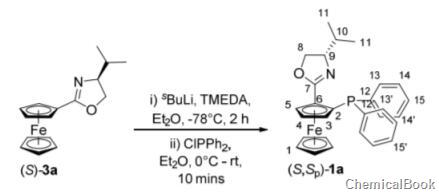
将恶唑啉(S)-3a(0.158 g,0.53 mmol)添加到在氩气气氛下火焰干燥的Schlenk管,并溶于无水乙醚(6毫升)。 加入四甲基乙二胺(0.100 ml,0.70 mmol),随后将橙色溶液冷却至-78℃并搅拌5分钟,然后将仲丁基锂(1.4 M)(0.500ml,0.70mmol)缓慢加入。搅拌2小时后,混合物将其加热至0℃并将新鲜蒸馏的氯二苯膦(0.120 ml,0.70 mmol)通过注射器添加。使反应升温至室温,然后再过10分钟,用乙醚稀释,然后用饱和碳酸氢钠水溶液淬灭。用水分离有机物,硫酸镁干燥,真空除去溶剂后得到粗产物。柱色谱法(SiO2,10%EtOAc的己烷溶液)洗脱为橙色固体非对映异构体(0.11 g,43%):
Rf0.23 (10 % EtOAc in hexane); νmax (film)/cm-1 3072, 3048,2955, 2934, 1658 (C=N); [α]D22.6°C = +92 (c = 0.1, EtOH);1H NMR (500 MHz, CDCl3) δ 7.51 -
7.46 (2H, m, o-Ph-H), 7.38-7.34 (3H, m, m+p-Ph-H), 7.25-7.17 (5H, m, o,m+p-Ph-H), 4.99(1H, brs, Cp-H), 4.37 (1H, t, J = 2.4 Hz, Cp-H), 4.26 (1H, dd, J = 9.4, 8.4 Hz, CHH), 4.22 (5H, s,CpH), 3.86 (1H, ddd, J = 9.5, 8.0, 5.7 Hz, CH), 3.68 (1H, t, J = 8.2 Hz, CHH), 3.63 - 3.59 (1H, m,Cp-H), 1.71 - 1.63 (1H, m, iPr(H)), 0.82 (3H, d, J = 6.8 Hz, iPr(Me)), 0.69 (3H, d, J = 6.8 Hz,iPr(Me)); 13C NMR (125 MHz, CDCl3) δ 165.4 (C7), 139.7 (d, J = 12.8 Hz, C12), 138.3 (d, J = 13.4Hz, C12’), 135.0 (d, J = 21.4 Hz, C13’), 132.6 (d, J = 19.4 Hz, C13), 129.1 (C15’), 128.3 (d, J = 7.2Hz, C14’), 128.2 (d, J = 6.8 Hz, C14), 128.0 (C15), 78.72 (d, J = 15.2 Hz, C2), 75.5 (d, J = 18.4 Hz,C6), 74.0 (d, J = 4.1 Hz, C3), 72.3 (C5), 72.2 (C9), 70.9 (C1+4), 69.8 (C8
), 29.9 (C10), 18.8 (C11’),17.7 (C11); 31P NMR (202 MHz, CDCl3) δ -16.87 (PPh2).
主要参考资料
[1]From Organic Letters, 19(3), 702-705; 2017
