简介
我国目前由于对Fmoc-O-叔丁基-D-酪氨酸应用研究不多,酪氨酸尚未得到充分开发利用,在18种氨基酸中可以说酪氨酸类衍生物是应用最少的种类。因此,国内市场上Fmoc-O-叔丁基-D-酪氨酸几乎不占什么份额,供大于求[1]。虽然一些大型氨基酸生产企业有每月数十吨的生产能力,可能实际上也没有生产那么多Fmoc-O-叔丁基-D-酪氨酸产品上市。尽管我国Fmoc-O-叔丁基-D-酪氨酸市场供大于求,但仍有一些生化制品公司出于商业利益考虑,仍从国外进口Fmoc-O-叔丁基-D-酪氨酸经分装后作为生化试剂销售,但就总量而言也是微不足道的。中国林科院亚林所经过多年研究,己经将竹副产品Fmoc-O-叔丁基-D-酪氨酸用酶促化学方法合成,不仅提高了酪氨酸产品的附加值,而且推动了竹产业的发展,开拓了与Fmoc-O-叔丁基-D-酪氨酸相关产品应用的新领域[2-4]。其结构式如图所示。
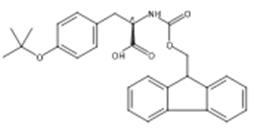
图1 Fmoc-O-叔丁基-D-酪氨酸的结构式。
应用
Fmoc-O-叔丁基-D-酪氨酸在医药上是合成甲状腺素、多巴、肾上腺素的前体,直接参与合成多巴胺、去甲肾上腺素和肾上腺素等神经递质,控制神经递质水平,参与甲状腺、脑垂体等腺体激素的合成[5]。临床上用于生殖避孕疗效研究。同时。Fmoc-O-叔丁基-D-酪氨酸可以促进蛋白质的合成和骨的钙化,对生长发育有很大的影响。它可通过血脑屏障,在脑中经多巴胺酶作用生成供机体维持能量的物质[6]。在临床上Fmoc-O-叔丁基-D-酪氨酸用于治疗肝昏迷、帕金森氏综合症、溃疡病、心力衰竭、痴呆症及精神病等证。国外已将Fmoc-O-叔丁基-D-酪氨酸用于肾脏病患者。例如:英国科学家将Fmoc-O-叔丁基-D-酪氨酸开发成一种制剂,服用这种制剂减肥时没有饥饿感而有幸福感,它能使人在身体和精神上产生兴奋[7]。随着我国医疗保健水平的提高,Fmoc-O-叔丁基-D-酪氨酸的需求量将会迅速增加。
事实上,Fmoc-O-叔丁基-D-酪氨酸主要供作合成神经递质(如多巴胺、去甲肾上腺素和肾上腺素等),这些物质关系到体力和精神状态[8]。科学家认为,抑郁症或精神消沉产生的直接原因是脑中多巴胺、去甲肾上腺素和其他神经递质含量水平降低,服用酪氨酸能够延长这些化合物的半衰期,也就自然能解脱精神和机体萎靡症状,从而能更好的工作而富于创造性[9]。大量的研究和试验都证明,服用Fmoc-O-叔丁基-D-酪氨酸增加了神经递质的合成,而且实际上也减轻了抑郁症症状,变得机敏和注意力集中[10]。
此外,在饲料工业中,Fmoc-O-叔丁基-D-酪氨酸可作为饲料添加剂,添加于雏禽的饲料中。一方面,尽量减少苯丙氨酸转化为酪氨酸的数量,以维护畜、禽生长中氨基酸的平衡。另一方面是避免雏鸡、仔猪因体内酪氨酸不足,甲状腺、肾上腺激素受到破坏而引起体重降低[11]。
治疗作用
服用Fmoc-O-叔丁基-D-酪氨酸实际表现出来的对精神、体能、代谢速率、皮肤、生长速率和心理的调节作用,表明Fmoc-O-叔丁基-D-酪氨酸在临床应用和保健功能是多方面的[12]。主要对长期疲劳、嗜睡症、烦闷、抑郁症、性冷淡、变态反应(过敏症)、头疼、抗饥饿、抗紫外辐射、减肥、血压、帕金森氏症、增强记忆、注意力缺陷混乱、痴呆症、苯酮服症、解酒、精神振奋等人群有很好的改善效果[13]。而且国外己将Fmoc-O-叔丁基-D-酪氨酸研制成药品和保健品,其中有Fmoc-O-叔丁基-D-酪氨酸粉剂、片剂、胶囊、维生素复合剂、Fmoc-O-叔丁基-D-酪氨酸-微量元素复合剂、和Fmoc-O-叔丁基-D-酪氨酸-多腺体复合剂[14-15]。
参考文献
[1] Y. Al-Abed, Synthesis and uses of peptidomimetics including azapeptides, The Feinstein Institutes for Medical Research, USA . 2020, p. 222pp.
[2] J. Bojarska, M. Remko, I.D. Madura, K. Kaczmarek, J. Zabrocki, W.M. Wolf, Synthesis, experimental and in silico studies of N-fluorenylmethoxycarbonyl-O-tert-butyl-N-methyltyrosine, coupled with CSD data: a survey of interactions in the crystal structures of Fmoc-amino acids, Acta Crystallogr., Sect. C: Struct. Chem. 76(4) (2020) 328-345.
[3] J.R. Courter, J.Z. Hamilton, N.R. Hendrick, M. Zaval, A.B. Waight, R.P. Lyon, P.D. Senter, S.C. Jeffrey, P.J. Burke, Structure-activity relationships of tubulysin analogues, Bioorg. Med. Chem. Lett. 30(14) (2020) 127241.
[4] T. Ishizawa, Preparation of O-alkyl serine derivatives by reacting serine cyclic sulfamidate derivatives with alcohols, Chugai Seiyaku Kabushiki Kaisha, Japan . 2020, p. 96pp.
[5] A. Jamieson, A. Mahindra, I. Black, Fmoc protected (2S)-2-amino-8-[(1,1-dimethylethoxy)amino]-8-oxo-octanoic acid, (S)-2-amino-8-oxononanoic acid and (S)-2-amino-8-oxodecanoic acid for peptide synthesis, The University Court of the University of Glasgow, UK . 2019, p. 58pp.
[6] S. Jiang, H. Hao, T. Wang, X. Wu, M. Wang, K. Zhang, Preparation method of oxadiazoles and thiadiazoles and their application, China Pharmaceutical University, Peop. Rep. China . 2019, p. 31pp.
[7] V. Kshtriya, B. Koshti, H. Narode, S. Naskar, N. Gour, Controlled self-assembly of modified aromatic amino acids, ChemRxiv (2021) 1-15.
[8] W. Li, X. Feng, J. Wang, M. Tian, L. Wen, S. Liang, B. Wang, Method for preparing N-(9-fluorenylmethyloxycarbonyl)-O-tert-butyl-L-serine, Sichuan Shifang Sangao Biochemical Industry Co., Ltd., Peop. Rep. China . 2019, p. 8pp.
[9] K. Nomura, R. Takeyama, Method for producing peptides, and method for deactivating bases used for deprotecting Fmoc group, Chugai Seiyaku Kabushiki Kaisha, Japan . 2019, p. 84pp.
[10] J. Park, S.-M. Jin, A.K. Mishra, J.A. Lim, E. Lee, Photo-Curable Lacquer Sap Resin Based on Urushiol-Mimicking, Tyrosine-Containing Additive, Langmuir 38(32) (2022) 10010-10021.
[11] M. Skoczylas, S. Bocian, B. Buszewski, Quantitative structure - retention relationships of amino acids on the amino acid- and peptide-silica stationary phases for liquid chromatography, J. Chromatogr. A 1609 (2020) 460514.
[12] H. Takeuchi, Y. Asaka, A. Nagaya, M. Handa, K. Masuya, T. Taguri, Y. Nemoto, Y. Kobayashi, A. Matsuda, H. Kurasaki, D.R. Cary, Method for producing peptide compounds containing N-alkyl amino acids by coupling of amino acid mixed anhydrides with silylated amino acids, Nissan Chemical Corporation, Japan; PeptiDream Inc. . 2020, p. 145pp.
[13] A. Temperini, D. Aiello, F. Mazzotti, C.M. Athanassopoulos, P. De Luca, C. Siciliano, 2,3-diaminopropanols obtained from D-serine as intermediates in the synthesis of protected 2,3-L-diaminopropanoic acid (L-Dap) methyl esters, Molecules 25(6) (2020) 1313.
[14] X. Xie, X. Suo, Y. Li, Q. Wang, Y. Jiang, Y. Cui, Oligopeptide two-dimensional nanomaterial and application as drug carriers, Xinzhou Teachers University, Peop. Rep. China . 2022, p. 16pp.
[15] J. Zhang, X. Pan, L. Liang, W. Lu, S. Wang, L. He, R. Si, J. Wang, Preparation of peptoid compounds containing tert-butyl substituted serine useful as Abl kinase inhibitors for the treatment of chronic myelocytic leukemia, Xi'an Jiaotong University, Peop. Rep. China . 2019, p. 15pp.
