| Company Name: |
|
| Tel: |
|
| Email: |
info@shivapharmachem.com |
| Products Intro: |
Cas:543-59-9
ProductName:Pentyl chloride
Purity: 99% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
|
| Company Name: |
|
| Tel: |
|
| Email: |
atul@kscl.co.in |
| Products Intro: |
Cas:543-59-9
ProductName:Pentyl chloride
Purity: 98% | Package: 1 kg,5 kg, 10 kg,25kg
|
| Company Name: |
|
| Tel: |
|
| Email: |
sohamchemicalindustries@gmail.com |
| Products Intro: |
Cas:75-65-0
ProductName:Tertiary Butanol
Purity: 99% | Package: 25KG;INR
|
Tertiary Pentyl Chloride manufacturers
|
| | Tertiary Pentyl Chloride Basic information |
| Product Name: | Tertiary Pentyl Chloride | | Synonyms: | 1,1-Dimethylpropyl chloride;1,1-dimethylpropylchloride;2-Chloro-2-methylbutane,95%;NSC 7900;2-Methyl-2-chlorobutane;3-chloroisopentane;butane,2-chloro-2-methyl-;Tertiary pentyl chloride | | CAS: | 594-36-5 | | MF: | C5H11Cl | | MW: | 106.59 | | EINECS: | 209-836-2 | | Product Categories: | Organics | | Mol File: | 594-36-5.mol | 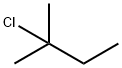 |
| | Tertiary Pentyl Chloride Chemical Properties |
| Melting point | -73 °C (lit.) | | Boiling point | 85-86 °C (lit.) | | density | 0.866 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.405(lit.) | | Fp | 15 °F | | form | clear liquid | | color | Colorless to Light yellow | | Water Solubility | Soluble in ethanol, ethyl ether. Insoluble in water. | | BRN | 1696930 | | Dielectric constant | 9.3000000000000007 | | Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents. | | LogP | 2.52 at 25℃ | | CAS DataBase Reference | 594-36-5(CAS DataBase Reference) | | EPA Substance Registry System | Butane, 2-chloro-2-methyl- (594-36-5) |
| Hazard Codes | F,Xi | | Risk Statements | 11-36/37/38 | | Safety Statements | 16-26-36 | | RIDADR | UN 1993 3/PG 2 | | WGK Germany | 3 | | TSCA | Yes | | HazardClass | 3 | | PackingGroup | II | | HS Code | 29031980 |
| | Tertiary Pentyl Chloride Usage And Synthesis |
| Chemical Properties | colourless liquid | | Uses | 2-Chloro-2-methylbutane is used as a solvent for the synthesis of other compounds and pentane. It is used in organic synthesis, Catalytic agent, Petrochemical additive. | | General Description | The influence of carbon-carbon multiple bonds on the solvolyses of 2-chloro-2-methylbutane has been critically evaluated through the extended Grunwald-Winstein equation. The rate constant for the SN 1 hydrolysis of 2-chloro-2-methylbutane has been measured near the consolute point of the liquid mixture of isobutyric acid and water. | | Purification Methods | Methods of purification commonly used for other alkyl chlorides lead to decomposition. Unsaturated contaminants are removed by chlorination with a small amount of chlorine in bright light, followed by distillation [Chien & Willard J Am Chem Soc 75 6160 1953]. [Beilstein 1 H 134, 1 I 46, 1 II 100, 1 III 357, 1 IV 324.] |
| | Tertiary Pentyl Chloride Preparation Products And Raw materials |
|