磺胺氯吡嗪
| 中文名称 | 磺胺氯吡嗪 |
|---|---|
| 中文同义词 | 磺胺氯吡嗪;N-(5-氯-3-吡嗪基)-4-氨基苯磺酰胺;磺胺氯吡嗪鈉;磺胺氯毗嗪钠;磺胺氯吡嗪(标准品);磺胺氯吡嗪溶液, 100PPM;磺胺氯吡嗪钠一水合物;磺胺氯吡嗪钠 100MG |
| 英文名称 | N-(5-CHLORO-3-PYRAZINE)-4-AMINOBENZENESULFONAININO |
| 英文同义词 | N-(5-CHLORO-3-PYRAZINE)-4-AMINOBENZENESULFONAININO SODIUM MONOHYDRATE;SULFACHLOROPYRAZINE SODIUM;SULFACHLOROPYRAZINE SODIUM H2O;SULFACHLORPYRAZINE SODIUM;sulfaclozine;SULFACLOZINE SODIUM;SPZ;SULFACHLOROPYRAZINE SODIUM(SPZ):N-(5-CHLORO-3-PYRAZINE)-4-AMINOBENZENESULFONAININO SODIUM MONOHYDRATE |
| CAS号 | 102-65-8 |
| 分子式 | C10H9ClN4O2S |
| 分子量 | 284.72 |
| EINECS号 | 203-044-0 |
| 相关类别 | 磺胺类产品;生命科学标准品;医药原料;中间体;兽药原料药;原料药;兽药;原料药;原料中间体-原料药;农用兽用原料;API原料药-抗菌;药物杂质及中间体;标准品 -中药标准品;兽药原料药;原料;添加剂;化工材料;医药、农药及染料中间体;Pharmaceutical intermediate;pharmaceutical;1;抗寄生虫;API原料药-兽药原料药;化学试剂-兽用原料;定制;化工原料;化学试剂 |
| Mol文件 | 102-65-8.mol |
| 结构式 | 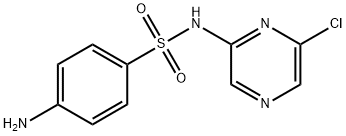 |
磺胺氯吡嗪 性质
| 熔点 | 234.8-235.4 °C |
|---|---|
| 沸点 | 495.7±55.0 °C(Predicted) |
| 密度 | 1.588±0.06 g/cm3(Predicted) |
| 储存条件 | 2-8°C(protect from light) |
| 溶解度 | DMSO(轻微)、甲醇(轻微、加热、超声处理) |
| 形态 | 固体 |
| 酸度系数(pKa) | 4.83±0.10(Predicted) |
| 颜色 | 白色至类白色 |
Bacterial; Parasite
The elimination of Sulfaclozine in the three systems: UV/TiO 2 , UV/K 2 S 2 O 8 , and UV/TiO 2 /K 2 S 2 O 8 . Sulfaclozine is weakly adsorbed on the surface of TiO 2 at pH 7 (< 5%) but efficiently eliminated with the following three systems: UV/TiO 2 , UV/K 2 S 2 O 8 , and UV/TiO 2 /K 2 S 2 O 8 in ultra pure water. Moreover, 12 of Sulfaclozine by-products are identified and reaction pathways show that, in addition of • OH and SO 4 •− radicals, the conduction-band electrons are responsible for the formation of some main by-products either directly or by the formation of superoxide radicals.
Sulfaclozine (60 mg/kg; intravenous injection or oral administration; male broiler chickens) can be used primarily for the treatment of parasitic and microbial infections of the digestive tract rather than for the treatment of systemic infections.
| Animal Model: | 14 male broiler chickens (30-day-old) |
| Dosage: | 60 mg/kg |
| Administration: | Intravenous injection or oral administration (Pharmacokinetic Analysis) |
| Result: | Serum drug concentrations at 0.083, 0.50, 2, 6, 24 and 72h were determined to be 99.62, 83.50, 72.68, 58.43, 38.66 and 13.14 μg/mL, respectively, by intravenous injection. By oral administration were determined as 4.33, 7.95, 16.46, 22.88, 16.03 and 5.74 μg/mL, respectively. |
