| Company Name: |
Hangzhou Huiyi Biotechnology Co., LTD
|
| Tel: |
0571-89918262 13357170655 |
| Email: |
chenlingwei@hizyme.com |
| Products Intro: |
Cas:23214-92-8
ProductName:Doxorubicin
Purity: HPLC90%纯度;HPLC90%纯度95% | Package: 1mg;10mg;100mg;1g
|
- Doxorubicin
-

- $0.00 / 1kg
-
2024-04-15
- CAS:23214-92-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
|
| Product Name: | Doxorubicin | | Synonyms: | 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S,10S)-;ADRIAMYCIN;14-Hydroxydaunomycin;adiblastine (hydrochloride salt);adriablastine (hydrochloride salt);adriablatina (hydrochloride salt);Adriacin (hydrochloride salt);Adriamycin PFS (hydrochloride salt) | | CAS: | 23214-92-8 | | MF: | C27H29NO11 | | MW: | 543.53 | | EINECS: | 245-495-6 | | Product Categories: | Isotope;NUFLOR;Antineoplastic/antibiotic;API | | Mol File: | 23214-92-8.mol |  |
| | Doxorubicin Chemical Properties |
| Melting point | 205°C | | Boiling point | 617.77°C (rough estimate) | | density | 1.3783 (rough estimate) | | refractive index | 1.6400 (estimate) | | storage temp. | Keep in dark place,Sealed in dry,2-8°C | | solubility | ≥27.2 mg/mL in DMSO; insoluble in EtOH; ≥24.8 mg/mL in H2O with ultrasonic | | form | solid | | pka | pKa 8.2 (Uncertain) | | Water Solubility | Soluble | | CAS DataBase Reference | 23214-92-8 | | IARC | 2A (Vol. 10, Sup 7) 1987 | | EPA Substance Registry System | Doxorubicin (23214-92-8) |
| HS Code | 2941900000 | | Hazardous Substances Data | 23214-92-8(Hazardous Substances Data) | | Toxicity | An anthracycline cytotoxic
antineoplastic that is produced by Streptomyces peucetius. The
LD50 in mice is 9.4 mg/kg, i.v. It is a carcinogen that inhibits
DNA and RNA synthesis by intercalating in double-stranded
DNA with the amino sugar in the minor groove and the 90-OH
group of the anthracycline ring hydrogen-bonded to the adjacent
guanine. It also alters membrane fluidity and ion transport, and
generates free radicals through a cytochrome P450-mediated
reductive process. In humans, it causes alopecia, stomatitis, nausea,
vomiting, diarrhea, cardiotoxicity (manifested by tachycardia),
and potentially fatal congestive heart failure. |
| Provider | Language |
|
(1S,3S)-3,5,12-Trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside
| English |
| | Doxorubicin Usage And Synthesis |
| Uses | Doxorubicin is in a class of medications called anthracyclines. It works by slowing or stopping the growth of cancer cells in your body. Doxorubicin is used in combination with other medications to treat certain types of the bladder, breast, lung, stomach, and ovarian cancer; Hodgkin's lymphoma (Hodgkin's disease) and non-Hodgkin's lymphoma (cancer that begins in the cells of the immune system); and certain types of leukemia (cancer of the white blood cells), including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML, ANLL).
Doxorubicin is also used alone and in combination with other medications to treat certain types of thyroid cancer and certain types of soft tissue or bone sarcomas (cancer that forms in muscles and bones). It is also used to treat neuroblastoma (a cancer that begins in nerve cells and occurs mainly in children) and Wilms' tumor (a type of kidney cancer that occurs in children). | | Mechanism of action |
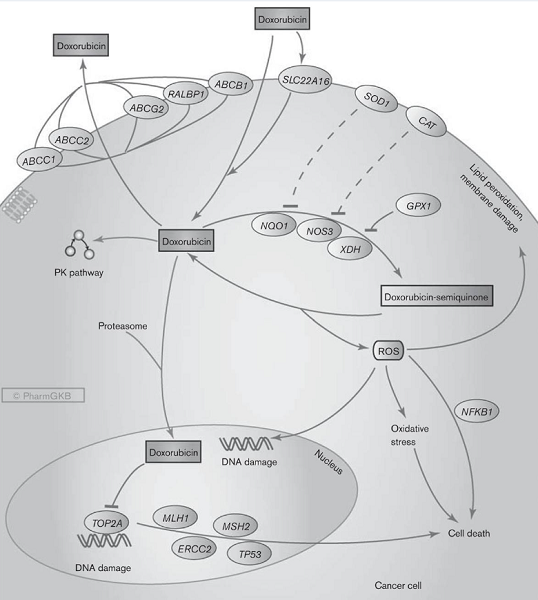
There are two proposed mechanisms by which doxorubicin acts in the cancer cell: (i) intercalation into DNA and disruption of topoisomerase-II-mediated DNA repair and (ii) generation of free radicals and damage to cellular membranes, DNA, and proteins. In brief, doxorubicin is oxidized to semiquinone, an unstable metabolite, which is converted back to doxorubicin in a process that releases reactive oxygen species. Reactive oxygen species can lead to lipid peroxidation and membrane damage, DNA damage, oxidative stress, and trigger apoptotic pathways of cell death. Candidate genes that may modulate this pathway involve those capable of the oxidation reaction (NADH dehydrogenases, nitric oxide synthases, xanthine oxidase) and those capable of deactivating the free radicals such as glutathione peroxidase, catalase, and superoxide dismutase. Alternatively, doxorubicin can enter the nucleus and poison topoisomerase-II, resulting in DNA damage and cell death.
| | Side effects | Adverse reactions are common after doxorubicin administration, including fatigue, alopecia, nausea and vomiting, and oral sores. Bone marrow suppression and an increased risk of secondary malignancy diagnoses may occur.
| | antibiotic | Doxorubicin is an antibiotic derived from the Streptomyces peucetius bacterium. It has been widely used as a chemotherapeutic agent since the 1960s. Doxorubicin is part of the anthracycline group of chemotherapeutic agents. Doxorubicin may be used to treat soft tissue and bone sarcomas and cancers of the breast, ovary, bladder, and thyroid. It also treats acute lymphoblastic leukemia, acute myeloblastic leukemia, Hodgkin lymphoma, and small-cell lung cancer.
|
| | Doxorubicin Preparation Products And Raw materials |
|