| Company Name: |
DC Chemicals
|
| Tel: |
021-58447131 13564518121 |
| Email: |
sales@dcchemicals.com |
| Products Intro: |
Cas:56-75-7
ProductName:Chloramphenicol (Chloromycetin)
Purity: 98% HPLC | Package: 100mg,250mg,1g
|
| Company Name: |
PhyCell Medical research Ltd
|
| Tel: |
138-18883417 13818883417 |
| Email: |
1281600188@qq.com |
| Products Intro: |
Cas:
ProductName:Chloromycetin CAP
Purity: >95%
|
| Company Name: |
DC Chemicals
|
| Tel: |
+86-21-58447131 |
| Email: |
sales@dcchemicals.com |
| Products Intro: |
Cas:56-75-7
ProductName:Chloramphenicol(Chloromycetin)
Brand:dcchemicals | Product Number:DCAPI1255 | Purity:>99%
|
Related articles - The utility of chloramphenicol
- Chloramphenicol was the first of the clinically useful antibiotics to be synthesized and the only one which is marketed in syn....
- Jun 27,2022
- What is Chloramphenicol?
- Chloramphenicol was originally isolated from Streptomyces venezuelae. It competes with transfer RNA at the peptidyl transferas....
- Mar 21,2022
|
| | Chloromycetin Chemical Properties |
| Melting point | 148-150 °C(lit.) | | Boiling point | 644.9±55.0 °C(Predicted) | | alpha | 19.5 º (c=6, EtOH) | | density | 1.6682 (rough estimate) | | refractive index | 20 ° (C=5, EtOH) | | Fp | 14 °C | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | absolute ethanol: soluble5-20mg/mL (as a stock solution) | | pka | 11.03±0.46(Predicted) | | form | powder | | color | white | | Water Solubility | 2.5 g/L (25 º C) | | Merck | 14,2077 | | BRN | 2225532 | | BCS Class | 3 | | InChIKey | WIIZWVCIJKGZOK-RKDXNWHRSA-N | | LogP | 1.140 | | CAS DataBase Reference | 56-75-7(CAS DataBase Reference) | | IARC | 2A (Vol. Sup 7, 50) 1990 | | NIST Chemistry Reference | Chloramphenicol(56-75-7) | | EPA Substance Registry System | Chloramphenicol (56-75-7) |
| | Chloromycetin Usage And Synthesis |
| Description | Chloramphenicol, also known as chlornitromycin, is a broad-spectrum, bacteriostatic antibiotic derived from Streptomyces venezuelae. It is first isolated from cultures of Streptomyces venequelae in 1947 but now produced synthetically. The synthetic product is racemic, also called synthomycin. Syntomycin is a mixture of chloramphenicol L-isomer and d-isomer. Because of dextroisomer antibacterial effect, the effect of synthomycin is only half of the natural products. It has a relatively simple structure and was the first broad-spectrum antibiotic to be discovered. It is effective against several gram-positive and gram-negative bacteria and commonly used in researching protein synthesis and to select for chloramphenicol-resistant transformed cells or the bacterial CAT gene.
Chloramphenicol is a semisynthetic, broad-spectrum antibiotic derived from Streptomyces venequelae with primarily bacteriostatic activity. Chloramphenicol diffuses through the bacterial cell wall and reversibly binds to the bacterial 50S ribosomal subunit. The binding interferes with peptidyl transferase activity, thereby prevents transfer of amino acids to the growing peptide chains and blocks peptide bond formation. As a result bacterial protein synthesis is blocked and impede bacterial cell proliferation.
| | Chemical Properties | It is white or yellowish green needle like crystals. The melting point is 150.5-151.5℃ (149.7-150.7℃). Under the high vacuum it can be sublimated, slightly soluble in water (2.5mg/ml at 25℃), slightly soluble in propylene glycol (150.8mg/ml), soluble in methanol, ethanol, butanol, ethyl acetate, acetone, insoluble in ether, benzene, petroleum ether, vegetable oil. Taste is very bitter.
| | Uses |
- Chloramphenicol is used for the treatment caused by typhoid bacillus, dysentery bacillus, Escherichia coli, bacillus, influenza and pneumococcal infections such as brucellosis.
- Chloramphenicol is used in the treatment of infections caused by bacteria. It works by killing bacteria or preventing their growth.
- Chloramphenicol is used to treat serious infections in different parts of the body. It is sometimes given with other antibiotics. However, chloramphenicol should not be used for colds, flu, other virus infections, sore throats or other minor infections, or to prevent infections.
- Chloramphenicol should only be used for serious infections in which other medicines do not work. This medicine may cause some serious side effects, including blood problems and eye problems. Symptoms of the blood problems include pale skin, sore throat and fever, unusual bleeding or bruising, and unusual tiredness or weakness.
You and your doctor should talk about the good this medicine will do as well as the risks of taking it .
Chloramphenicol is available only with your doctor's prescription.
| | Antimicrobial Spectrum | Chloramphenicol is bacteriostatic and a broad-spectrum antibiotic active against both gram-positive and gram-negative bacteria including rickettsia (cause of rocky-mountain spotted fever) and chlamydia. It is also found effective against Haemophilus influenzae causing meningitis.
- Gram-positive: Streptococcus spp., Staphylococcus spp., Enterococcus spp., Bacillus anthracis, Listeria monocytogenes.
- Gram-negative: Hemophilus influenzae, M. catarrhalis, N. meningitides, E. coli, P. mirabilis, Salmonella spp., Shigella spp., Stenotrophomonas maltophilia.
| | Mechanism of action | Inhibition of protein synthesis, Chloramphenicol irreversibly binds to a receptor site on the 50S subunit of the bacterial ribosome, inhibiting peptidyl transferase. This inhibition consequently results to the prevention of amino acid transfer to growing peptide chains, ultimately leading to inhibition of protein formation.
| | Pharmacokinetics | After oral administration, it is rapidly and completely absorbed, can be widely distributed in body tissues and body fluids. In the cerebrospinal fluid concentration distribution were higher than other antibiotics, oral bioavailability was 75%~90%. After oral half hour of in the blood can reach the effective concentration, It can reach the peak in 2 to 3 hours. Take oral 0.5g, 1g and 2g, blood drug concentration was 4mg/L, 8~10mg/L and 16~21mg/L in 2 hours, 1~2g, 4 times a day, can make the blood to maintain long-term effective concentration of 10mg/L~5. After intravenous injection, the average is similarity with oral blood drug concentration of the same dose. After intramuscular absorption is slow and irregular, blood concentration is only oral amounts of 50%, but the maintenance time is long. The plasma protein binding rate is 50%~60%., the half-life of 2 to 3 hours, the half-life of newborns was significantly higher than that of adults, under the age of 2 is about 24 hours, 2 to 4 years is about 12 hours. This product is absorbed and widely distributed in the body each Reduce the concentrations of blood concentration in the blood to body fluids and tissues of liver and kidney was the highest, followed by the lung, spleen, heart, intestine and brain. Bile content is low, about 20%~50%, but also can enter the pleural effusion and ascites, milk, fetal circulation and ocular tissue. Through the blood brain barrier to reach the cerebrospinal fluid (CSF), in normal cerebrospinal fluid (CSF) in concentrations is up to 20%~50%, inflammation is up to 50%~100%. It is mainly in the liver metabolism, binding with glucuronic acid to inactivation, about 75%~90% of the metabolites in 24 hours urine, of which 5%~15% for the prototype drug. 1 g orally, urine concentration is 70~150mg/L. serious liver disease patients, the half-life may be extended due to hepatic metabolism and poisoning caused by accumulation.
| | Indications | This product is fat soluble, synthetic peptide can inhibit the formation and prevent protein. Is a bacteriostatic agent, high concentration or effect highly sensitive to the bacteria to this product showed bactericidal action. This product is applicable to the general application of typhoid and paratyphoid salmonella, and other Bacteroides fragilis.
1. Chloramphenicol is the preferred treatment of typhoid and paratyphoid fever, and can be used for typhoid Salmonella infections.
2. Used for pneumococcal in patients allergic to penicillin, ampicillin B Hib meningitis or tolerance, meningococcal meningitis, sensitive to the change of gram negative bacilli meningitis.
3. Used for aerobic and anaerobic bacteria mixed infection of otogenic brain abscess.
4. Used for serious anaerobic infections, such as Bacteroides fragilis infection.
5. Used as aminoglycoside drugs in the treatment of infection caused by sensitive bacteria and other microorganisms, such as influenza bacillus, Salmonella and other gram negative bacilli to sepsis, pulmonary infection.
6. Used for the local treatment of flu from Escherichia coli, bacillus, Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus and eye, ear superficial infection.
7. It is effective for Rickettsia, mycoplasma, and infection.
8. Coli and Serratia of Pseudomonas aeruginosa is infections.
9. Chloramphenicol eye drops for the treatment of infections caused by sensitive bacteria caused by the eye, such as trachoma, conjunctivitis, keratitis, blepharitis etc..
10. This medicine local ear drops can be used for treating sensitive bacteria infections caused by otitis externa, acute and chronic otitis media, the drug ear plugs can also be for inflammation of the ear canal and radical mastoidectomy postoperative oozing pus.
| | resistance | Drug resistance of gram negative bacilli on chloramphenicol acetyltransferase, mostly due to drug inactivation, the enzyme is mediated by the R factor. Drug resistance of gram positive bacteria, may also be due to similar mechanisms, but not fully clear. Some strains of Pseudomonas aeruginosa and Proteus, Klebsiella, are another way to generate drug resistance, which prompted the permeability change, and chloramphenicol cannot enter the cell.
Bacterial resistance to chloramphenicol resistant strains both in vitro and in vivo, increased gradually in recent years. Escherichia coli, Salmonella and other gram negative bacilli can be due to drug resistance factor factor (R) transfer and acquire resistance. It has been proven that with R factor of Escherichia coli can produce acetyl transferase, chloramphenicol acetylation of failure; chloramphenicol resistant Staphylococcus aureus can also produce some inducible enzyme, under the participation of acetyl CoA, the acetylation of chloramphenicol.
| | Drug interactions | Chloramphenicol inhibits hepatic microsomal enzyme of phenytoin and tolbutamide (Jia Tangning) and chlorpropamide and dicoumarol (and possibly other drug metabolism, and the prolonged in vivo half-life, increased concentrations of serum. Poisoning aggravate that death is also reported. On the other side, phenobarbital, phenytoin, rifampin (are, 1985) can decrease the serum concentration of chloramphenicol, which was estimated to be due to the inductive effect of drugs on the liver enzymes. Therefore, at the same time, the application effect of chloramphenicol pharmacokinetics of drugs, should be paid attention to the monitoring of serum concentration of chloramphenicol.
Chloramphenicol can delay the iron, folic acid and vitamin B12 on anemia treatment response. It can interfere with the host to tetanus toxoid anamnestic response. Therefore, at the same time, the application situation chloramphenicol and active immune agents should be avoided.
Antagonistic effects of chloramphenicol on penicillin bactericidal effect, which is verified in vitro and animal experiments, but its clinical significance is not clear. This kind of combination, only in the proof of such treatment is benefit, began to be used.
| | Adverse reactions and precautions | 1. Inhibition of bone marrow hematopoietic function: for the most serious toxicity of chloramphenicol, such as red blood cells, granulocyte and platelet reduce. There are two types: one is the reversible inhibition, manifested as neutropenia and thrombocytopenia, and anemia, related to dose and duration can be gradually recovered after drug withdrawal; Second is irreversible aplastic anemia, with dosage and duration without direct relationship, low incidence, once often occur difficult to reverse, high mortality rate and a few survivors can development for granulocytic leukemia, women, children, and liver and kidney function not entire occurrence rate is high. This is the main reason to limit the clinical application.
2. In the liver, metabolism rate of this product is very high, on the function of the liver, it has impaired the appropriate quantity (adult day not more than 1 g) or not as much as possible.
3. Chloramphenicol is only 5%~10% prototype drug discharge from the kidney, it is not appropriate for the treatment of urinary tract infection.
4. Premature infants and neonatal as much as possible.
5. I see the spirit of neurological symptoms, should be promptly discontinued.
6. During late pregnancy and lactation are not suitable for this application, because this product in vivo is conjugated with glucuronic acid and detoxification, conjugates excreted by the kidneys. And this product can be through the placenta to the fetus, fetal and neonatal, due to not perfect of enzyme system in the liver, glucuronic acid combined with the ability is poor and excretory function of the kidney is weak. Therefore, it is very easy to cause drug accumulation, the newborn gray baby syndromes. Gray cyanosis, dyspnea, vomiting, abdominal distension and circulatory failure with unique performance, high fatality rate. In addition, this product can damage the hematopoietic system, can make the pregnant women aplastic anemia, neonatal thrombocytopenia and other consequences.
| | Methods of production | Methods for producing countries in the world to chloramphenicol had a lot of research, summed up: (1) p-nitroacetophenone method; (2) styrene method; (3) Cinnamyl alcohol method; (4) The nitro cinnamic alcohol method; (5) P-nitrobenzaldehyde method. China use p-nitroacetophenone method, the method is by ethylbenzene via nitration, oxidation, bromide, salt, hydrolysis, acetylation, addition, reduction, decomposition, split second chloride acetylation and chloramphenicol.
| | Description | Chloramphenicol was originally produced by fermentation of Streptomyces venezuelae, but its comparatively
simple chemical structure soon resulted in several efficient total chemical syntheses. With two asymmetric
centers, it is one of four diastereomers, only one of which (1R,2R) is significantly active. Because total
synthesis produces a mixture of all four, the unwanted isomers must be removed before use.
Chloramphenicol is a neutral substance that is only moderately soluble in water, because both nitrogen
atoms are nonbasic under physiologic conditions (one is an amide and the other a nitro moiety). It was the
first broad-spectrum oral antibiotic used in the United States and was once very popular. Severe
potential blood dyscrasia has greatly decreased its use in North America. Although its cheapness and
efficiency makes it still very popular in much of the rest of the world where it can often be purchased
over-the-counter without a prescription | | Chemical Properties | White to grey-white crystalline powder | | Chemical Properties | Chloramphenicol is a white to grayish-white
or yellowish-white crystalline solid. | | Originator | Leukomycin,Bayer,W. Germany | | Uses | antibacterial, antirickettsial, inhibits protein synthesis | | Uses | Broad spectrum antibiotic obtained from cultures of the soil bacterium Streptomyces venezuelae. It has a broad spectrum of activity against Gram-positive and gram-negative bacteria. Antibacterial; antirickettsial | | Uses | Chloramphenicol is unusual nitroaromatic metabolite produced by Streptomyces venezuelae, first published in 1947. Chloramphenicol is a broad spectrum antibiotic with good activity against Gram negative and anaerobic bacteria. Although restricted to ocular use, antibiotic resistance to other classes has refocused attention on this class. Chloramphenicol acts by binding to the 23S sub-unit of the 50S ribosome, inhibiting protein synthesis. Chloramphenicol has been extensively studied with over 35,000 literature citations. | | Definition | ChEBI: Chloramphenicol is an organochlorine compound that is dichloro-substituted acetamide containing a nitrobenzene ring, an amide bond and two alcohol functions. It has a role as an antimicrobial agent, an antibacterial drug, a protein synthesis inhibitor, an Escherichia coli metabolite, a Mycoplasma genitalium metabolite and a geroprotector. It is an organochlorine compound, a diol, a C-nitro compound and a carboxamide. | | Indications | Resistance to chloramphenicol is usually explained by the presence of a plasmid that
determines the production of chloramphenicol acetyltransferase. This enzyme acetylates
the drug, giving it unable to bind with 50 S subunits of bacterial ribosomes.
Chloramphenicol is a potentially toxic drug and has a few indications for use. It is the drug
of choice for treating typhoid fever, and it is used for treating brain abscesses. Until
recently, it was the drug of choice for therapy of bacterial meningitis in children (in com�bination with ampicillin). However, third-generation cephalosporins are currently pre�ferred for such purposes. Chloramphenicol is an effective alternative for a number of
infections in situations, where drugs of choice cannot be used for one reason or another.
However, it should never be used for infections that can readily be treated with other
antimicrobial drugs. Synonyms of this drug are levomycetin, amindan, aquamycetin,
chloromycetin, ophthoclor, opulets, leukomycin, and many others. | | Manufacturing Process | Chloramphenicol may be prepared by fermentation or by chemical synthesis.
The fermentation route to chloramphenicol is described in US Patents
2,483,871 and 2,483,892. To quote from US Patent 2,483,892: The cultivation
of Streptomyces venezuelae may be carried out in a number of different ways.
For example, the microorganism may be cultivated under aerobic conditions
on the surface of the medium, or it may be cultivated beneath the surface of
the medium, i.e., in the submerged condition, if oxygen is simultaneously
supplied.
Briefly stated, the production of chloramphenicol by the surface culture
method involves inoculating a shallow layer, usually less than about 2 cm, of a
sterile, aqueous nutrient medium with Streptomyces venezuelae and
incubating the mixture under aerobic conditions at a temperature between
about 20° and 40°C, preferably at room temperature (about 25°C), for a
period of about 10 to 15 days. The mycelium is then removed from the liquid
and the culture liquid is then treated by methods described for isolating therefrom the desired chloramphenicol.
The synthetic route to chloramphenicol is described in US Patent 2,483,884 as
follows: 1.1 g of sodium is dissolved in 20 cc of methanol and the resulting
solution added to a solution of 5 g of benzaldehyde and 4.5 g of beta�nitroethanol in 20 cc of methanol. After standing at room temperature for a
short time the gel which forms on the mixing of the reactants changes to a
white insoluble powder. The precipitate is collected, washed with methanol and
ether and then dried. The product thus produced is the sodium salt of 1-
phenyl-2-nitropropane-1,3-diol.
Eighteen grams of the sodium salt of 1-phenyl-2-nitropropane-1,3-diolis
dissolved in 200 cc of glacial acetic acid. 0.75 g of palladium oxide
hydrogenation catalyst is added and the mixture shaken at room temperature
under three atmospheres pressure of hydrogen overnight. The reaction vessel
is opened, 2.5 g of 10% palladium on carbon hydrogenation catalyst added
and the mixture shaken under three atmospheres pressure of hydrogen for 3
hours. The catalyst is removed from the reaction mixture by filtration and the
filtrate concentrated under reduced pressure. Fifty cubic centimeters of n�propanol is added to the residue and the insoluble inorganic salt removed by
filtration.
The filtrate is treated with excess hydrochloric acid and evaporated to obtain a
pale yellow oil. Five grams of the oil thus obtained is treated with 15 cc of
saturated potassium carbonate solution and the mixture extracted with 50 cc
of ether, then with 30 cc of ethyl acetate and finally with two 30 cc portions of
ethanol. Evaporation of the solvent from the extract gives the following
quantities of the desired 1-phenyl-2-aminopropane-1,3-diol: 0.5 g, 1.0 g and
3.1 g.
1.7 g of 1-phenyl-2-aminopropane-1,3-diol is treated with 1.6 g of methyl
dichloroacetate and the mixture heated at 100°C for 1.25 hours. The residue
is washed with two 20 cc portions of petroleum ether and the insoluble
product collected. Recrystallization from ethyl acetate yields the desired (dl)-
reg.-1-phenyl-2-dichloroacetamidopropane-1,3-diol in pure form; MP 154° to
156°C.Five hundred milligrams of (dl)-reg.-1-phenyl-2-dichloroacetamidopropane-
1,3-diolis added to a solution consisting of 1 cc of pyridine and 1 cc of acetic
anhydride and the resulting reaction mixture heated at 100°C for 1/2 hour.
The reaction mixture is evaporated to dryness under reduced pressure and the
residue taken up in and crystallized from methanol. Recrystallization from
methanol produces the pure diacetate of (dl)-reg.-1-phenyl-2-dichloro�acetamidopropane-1,3-diol (MP 94°C).
Two hundred milligrams of the diacetate of (dl)-reg.-1-phenyl-2-
dichloroacetamidopropane1,3-diol is added to a mixture consisting of 0.25 cc
of concentrated nitric acid and 0.25 cc of concentrated sulfuric acid at 0°C.
The reaction mixture is stirred until solution is complete, poured onto 25 g of
ice and the mixture extracted with ethyl acetate. The ethyl acetate extracts
are evaporated under reduced pressure and the diacetate of (dl)-reg.-1-
pnitrophenyl-2-dichloroacetamidopropane-1,3-diol so produced purified by
recristallization from ethanol; MP 134°C.
Five hundred milligrams of the diacetate of (dl)-reg.-1-p-nitrophenyl-2-
dichloroacetamidopropane-1,3-diol is dissolved in a mixture consisting of 25
cc of acetone and an equal volume of 0.2 N sodium hydroxide solution at 0°C
and the mixture allowed to stand for one hour. The reaction mixture is
neutralized with hydrochloric acid and evaporated under reduced pressure to
dryness. The residue is extracted with several portions of hot ethylene
dichloride, the extracts concentrated and then cooled to obtain the crystalline
(dl)-reg.-1- p-nitrophenyl-2-dichloroacetamidopropane-1,3-diol; MP 171°C. | | Brand name | Chloromycetin
(Parke-Davis);Acne-sol;Acnoxin;Actimac;Actinac;Alficetyn susp.;Altabactin;Ambrasynth;Amphemycin-prednisonum;Ampliomicetin;Amseclim;Angimidone;Angiters;Antibiopto;Aquapred;Armacol;Arrlicetin;Aviatrin;Balkamycin;B-cpct;Bemacol;Berlicetin;Biofeniol;Biophtas;Biotocap;Bismophenyl;Bitencyl;C. o fluo-fenicol;C. o hidrocor-clora;Cafenolo;Calmina;Campiol;Caosol;Cavumycetina;Ccombinado balsamico;Ccorticol;Cebenicol;Chemibal;Chemyzin;Chloramfenicol;Chloramol;Chloramphenicol intervetra;Chloramphenicol-pos;Chloramphycin;Chloramplast;Chloramson;Chloranfeni-mck;Chloranfeni-opipno;Chloranfeni-otico;Chloranfeni-ungena;Chloreptic;Chlorical;Chloroantibion;Chlorocortal;Chlorofair;Chloroject s;Chloromex;Chloromik;Chloromimyxin;Chloromycetin kapseals;Chloromycetin palmitate;Chloroptic p. oint.;Chlorostrep;Clorbiotina;Clorbis supp.;Cloromicetin;Cloromycetin;Cloroptic farmicetina;Clorosyntex;Colidene;Colimy-c;Cortican;Cortidermale;Cortimisin;Cortiphenicol;Cortison-quemicet;Cortivert;Cutispray no. 4;Cyphenicol;Cysticat;Davuron sedante;Dectamicina;Delta optil;Devamycetin;Dexa-biofinicol;Dorsec;Duphenicol;Econoclor;Ejificol strept;Ejificol sulfa;Elase chloromycel;Enttocetrin;Erittronicol;Erteilen;Esterofenil;Estevecicina cloranfenico;Extracicilina;Fago-praxin;Fluorobioptal;Furacol l;Furamecetil alpha magna;Furamecetil magna;Furatrimon;Furokatin;Gammaphenicol;Ginetris;Gino-dectacil;Gliscol;Globveticol;Goticas;Nova-phenicol;Novoclorocap;Oftan;Ophthaphenicol;Opthalon;Oralmisetin;Otiprin;Otopred ear drops;Pantofenicol;Parcyclin;Pedimycetin;Pentocetina;Pertaril;Pimabiciron;Pinimentac;Plastoderma;Prednomycetine;Procusulf;Protercicline;Prurivet;Pulmo vinco;Quitrase antibiotico;Ranphenicol;Ranstrepcol;Reclor;Redidropsol;Renegen;Reocetin;Reostop;Rheofin;Rivomycin sulfa;Rolintrex;Roncovita;Ronphenil;Roscomycin;Rovictor;Samaphenicol;Scanicoline;Scieramycetin;Sergo-amigdalar;Serviclofen;Sigmicilina;Sintomitsin;Snophenicol;Soludectancil;Sopamycetin;Spasmo-paraxin;Spersanicol;Strepticine;Streptoglobenicol;Streptophenicol;Subital supp.;Suismycetin;Sulfaglobenicol;Sulfamycetin;Synthomycetina;Synthophtone;Tardomyocel;Tega-cetin;Tetrachlorasone;Tetracol;Tetranfen;Tetraphenicol;Tetra-phenicol oculos;Tiframilk;Tiromycetin;Toramin;Transicetina;Transpulmycin;Tribiotic;Trophen;Troymycetin;Tusolone;Tycloran;Uro-gliscal 500;Uroletten-s;Uroplex 4;Ut forte;Uvomycin;Variolan;V-crayolan;Vetical;Vetophenicol;Viceton;Viklorin;Virogin;Vitaklorin;Vsmpozim;Wintetil;Zoppib spray blu;Gotimycetin;Ichthoseptal;Iruxolum;Isicetina;Isopto fenicol;Kavipe;Kloramfex. | | Therapeutic Function | Antimicrobial | | World Health Organization (WHO) | Chloramphenicol, an antibiotic isolated from Streptomyces
venezuelae in 1947, first became available for general clinical use in 1948. By 1950
it was evident that its use could cause serious, sometimes fatal, blood dyscrasias.
However, it remains one of the most effective antibiotics for treating invasive
typhoid fever and salmonellosis, some rickettsioses and serious infections caused
by Haemophilus influenzae or anaerobic organisms. This is considered to justify its
retention in the WHO Model List of Essential Drugs.
(Reference: (WHTAC1) The Use of Essential Drugs, 2nd Report of the WHO Expert
Committee, 722, , 1985) | | Antimicrobial activity | It is active against a very wide range of organisms. Minimum inhibitory concentrations (MICs) (mg/L) for other
organisms are: Staphylococcus epidermidis, 1–8; Corynebacterium
diphtheriae, 0.5–2; Bacillus anthracis, 1–4; Clostridium perfringens,
2–8; Mycobacterium tuberculosis, 8–32; Legionella pneumophila,
0.5–1; Bordetella pertussis, 0.25–4; Brucella abortus,
1–4; Campylobacter fetus, 2–4; Pasteurella spp., 0.25–4; Serratia
marcescens, 2–8; Burkholderia pseudomallei, 4–8. Most Gramnegative
bacilli are susceptible, but Pseudomonas aeruginosa
is resistant. Leptospira spp., Treponema pallidum, chlamydiae,
mycoplasmas and rickettsiae are all susceptible, but Nocardia
spp. are resistant. It is widely active against anaerobes, including
Actinomyces israelii (MIC 1–4 mg/L), Peptostreptococcus
spp. (MIC 0.1–8 mg/L), and Fusobacterium spp. (MIC 0.5–2
mg/L), but Bacteroides fragilis is only moderately susceptible
(MIC about 8 mg/L).
It is strictly bacteristatic against almost all bacterial species,
but exerts a bactericidal effect at 2–4 times the MIC
against some strains of Gram-positive cocci, Haemophilus
influenzae and Neisseria spp. The minimum bactericidal
concentrations (MBCs) for penicillin-resistant pneumococci
are often significantly higher than those for penicillin-
susceptible strains, although this cannot be detected
by conventional disk susceptibility testing or MIC determination.
Its bacteristatic effect may inhibit the action of
penicillins and other β-lactam antibiotics against Klebsiella
pneumoniae and other enterobacteria in vitro, but the clinical significance of this is doubtful. The presence of ampicillin
does not affect the bactericidal effect of chloramphenicol
on H. influenzae. | | Acquired resistance | The prevalence of resistant strains in many Gram-positive
and Gram-negative organisms reflects usage of the antibiotic.
Over-the-counter sales are believed to have compounded the
problem in some countries. For example, it has long been the
drug of choice for the treatment of typhoid and paratyphoid
fevers, but widespread use led to a high prevalence of resistant
Salmonella enterica serotype Typhi. Outbreaks of infection
caused by chloramphenicol-resistant S. Typhi have been
seen since the early 1970s. Use of co-trimoxazole and fluoroquinolones
in typhoid has resulted in a decline in chloramphenicol
resistance in some endemic areas. Many hospital
outbreaks caused by multiresistant strains of enterobacteria,
notably Enterobacter, Klebsiella and Serratia spp., have been
described.
Plasmid-borne resistance was first noted in shigellae in
Japan and subsequently spread widely in Central America,
where it was responsible for a huge outbreak. Strains of
S. Typhi resistant to many antibiotics including chloramphenicol
are particularly common in the Indian subcontinent.
Resistance in shigellae is also relatively common in
some parts of the world.
Resistant strains of H. influenzae (some also resistant to
ampicillin), Staph. aureus and Streptococcus pyogenes are also
encountered. Most N. meningitidis strains remain susceptible,but high-level resistance (MIC >64 mg/L) due to the production
of chloramphenicol acetyltransferase has been
described; the nucleotide sequence of the resistance gene was
indistinguishable from that found on a transposon in Cl. perfringens.
Resistant strains of Enterococcus faecalis are relatively
common, and resistance to chloramphenicol is found in some
multiresistant pneumococci.
Resistance in Staph. aureus is caused by an inducible
acetyltransferase; additionally, the cfr (chloramphenicol–
florfenicol
resistance) gene encodes a 23S rRNA methyltransferase
that also confers resistance to linezolid. In Escherichia
coli, the capacity to acetylate chloramphenicol (at least three
enzymes are involved) is carried by R factors. Replacement of
the 3-OH group, which is the target of acetylation, accounts
for the activity of fluorinated analogs against strains resistant
to chloramphenicol and thiamphenicol. The resistance
of B. fragilis and some strains of H. influenzae is also due
to elaboration of a plasmid-encoded acetylating enzyme;
in others it is due to reduced permeability resulting from
loss of an outer membrane protein. Some resistant bacteria
reduce the nitro group or hydrolyze the amide linkage.
Resistance of Ps. aeruginosa is partly enzymic and partly due
to impermeability. | | General Description | Synthetic bacteriostatic antibiotic that inhibits the translation of RNA by blocking the peptidyltransferase reaction on ribosomes. | | Hazard | Has deleterious and dangerous side effects.
Must conform to FDA labeling requirements. Use
is closely restricted. Probable carcinogen. | | Biochem/physiol Actions | Potency: ≥970 μg/mg | | Contact allergens | This broad spectrum phenicol group antibiotic has been
implicated in allergic contact dermatitis. Cross-sensitivity
to thiamphenicol is possible, but not systematic. | | Mechanism of action | Chloramphenicol is bacteriostatic by virtue of inhibition of protein biosynthesis in both bacterial and, to a
lesser extent, host ribosomes. Chloramphenicol binds to the 50S subparticle in a region near where the
macrolides and lincosamides bind.
Resistance is mediated by several R-factor enzymes that catalyze acetylation of the secondary and, to some
extent, the primary hydroxyl groups in the aliphatic side chain. These products no longer bind to the
ribosomes and so are inactivated. Escherichi a coli frequently is resistant because of chloramphenicol's lack
of intercellular accumulation. | | Pharmacokinetics | Oral absorption: 80–90%
Cmax 500 mg oral: 10–13 mg/L after 1–2 h
Plasma half-life: 1.5–3.5 h
Volume of distribution: 0.25–2 L/kg
Plasma protein binding: c. 25–60%
Absorption
The plasma concentration achieved is proportional to the
dose administered. Suspensions for oral administration to
children contain chloramphenicol palmitate, a tasteless and
bacteriologically inert compound, which is hydrolyzed in the
gut to liberate chloramphenicol. Following a dose of 25 mg/kg,
peak plasma levels around 6–12 mg/L are obtained, but there
is much individual variation.
Pancreatic lipase is deficient in neonates and, because of
poor hydrolysis, the palmitate should be avoided. In very young
infants, deficient ability to form glucuronides, and low glomerular
and tubular excretion greatly prolong the plasma half-life.
For parenteral use, chloramphenicol sodium succinate,
which is freely soluble and undergoes hydrolysis in the tissues
with the liberation of chloramphenicol, can be injected intravenously
or in small volumes intramuscularly. The plasma
concentrations after administration by these routes are unpredictable,
and approximate to only 30–70% of those obtained
after the same dose by the oral route. Protein binding is
reduced in cirrhotic patients and neonates, with correspondingly
elevated concentrations of free drug.
Distribution
Free diffusion occurs into serous effusions. Penetration
occurs into all parts of the eye, the therapeutic levels in the
aqueous humor being obtained even after local application
of 0.5% ophthalmic solution. Concentrations obtained in
cerebrospinal fluid (CSF) in the absence of meningitis
are 30–50% of those of the blood and greater in brain. It
crosses the placenta into the fetal circulation and appears
in breast milk.
Metabolism
It is largely inactivated in the liver by conjugation with
glucuronic acid or by reduction to inactive arylamines; clearance
of the drug in patients with impaired liver function is
depressed in relation to the plasma bilirubin level. It has been
suggested that genetically determined variance of hepatic
glucuronyl transferase might determine the disposition and
toxicity of the drug.
Excretion
It is excreted in the glomerular filtrate, and in the newborn
elimination may be impaired by the concomitant administration
of benzylpenicillin, which is handled early in life by the
same route. Inactive derivatives are eliminated partly in the
glomerular filtrate and partly by active tubular secretion. Over
24 h, 75–90% of the dose appears in the urine, 5–10% in biologically
active forms and the rest as metabolites, chiefly as
a glucuronide conjugate. Excretion diminishes linearly with
renal function and at a creatinine clearance of <20 ml/min,
maximum urinary concentrations are 10–20 mg/L rather
than the 150–200 mg/L found in normal subjects. Because
of metabolism, blood levels of active drug are only marginally
elevated in renal failure, but microbiologically inactive metabolites
accumulate. The plasma half-life of the products in the
anuric patient is around 100 h, and little is removed by peritoneal
or hemodialysis. Dosage modification is normally unnecessary
in renal failure as the metabolites are less toxic than
the parent compound. About 3% of the administered dose is
excreted in the bile, but only 1% appears in the feces, and this
mostly in inactive forms.
Interactions
Induction of liver microsomal enzymes, for example by
phenobarbital (phenobarbitone) or rifampicin (rifampin),
diminishes blood levels of chloramphenicol; conversely,
chloramphenicol, which inhibits hepatic microsomal oxidases,
potentiates the activity of dicoumarol (dicumarol), phenytoin,
tolbutamide and those barbiturates that are eliminated
by metabolism. It also depresses the action of cyclophosphamide,
which depends for its cytotoxicity on transformation
into active metabolites. It is uncertain whether this interaction may lead to a clinically significant level of inhibition of the
activity of cyclophosphamide. The half-life of chloramphenicol
is considerably prolonged if paracetamol (acetaminophen)
is given concurrently, and co-administration of these drugs
should be avoided. | | Clinical Use | Typhoid fever and other severe infections due to salmonellae
Rickettsial infections
Meningitis
Invasive infection caused by H. influenzae
Destructive lung lesions involving anaerobes
Eye infections (topical)
Reference is made to its use in cholera, plague, tularemia and
bartonellosis, melioidosis, Whipple’s disease and relapsing fever.
In enteric fever in adults, fluoroquinolones are associated with a
lower clinical relapse rate. Treatment for other serious infections
should be restricted to organisms that are resistant or much less
susceptible to other antibiotics. A study in low resource countries
found ampicillin plus gentamicin superior to injectable
chloramphenicol for the treatment of very severe communityacquired
pneumonia in children.
It has been used with varying success to treat infections
caused by glycopeptide-resistant enterococci. Meningitis
caused by penicillin-resistant pneumococci responds poorly,
apparently due to failure to achieve bactericidal concentrations
in CSF. It should never be given systemically for minor
infections. Topical use in the treatment of eye infections is
controversial given the unsubstantiated risk of bone marrow
aplasia. A placebo-controlled study in children with infective
conjunctivitis in the community found no clinical benefit in
the use of chloramphenicol eye drops.
The daily dose should not normally exceed 2 g, and the
duration of the course should be limited (e.g. 10 days).
Although patients may show toxic manifestations after receiving
very little drug, the danger is almost certainly increased by
excessive or repeated dosage or by the treatment of patients
with impaired hepatic or renal function, including those at the
extremes of life. The wide pharmacokinetic variability of the
antibiotic in neonates makes monitoring of serum concentrations
advisable. Determination of full blood counts should be
carried out twice weekly. | | Side effects | Glossitis, associated with overgrowth of Candida albicans,
is fairly common if the course of treatment exceeds 1 week.
Stomatitis, nausea, vomiting and diarrhea may occur,
but are uncommon. Hypersensitivity reactions are very
uncommon. Jarisch–Herxheimer-like reactions have been
described in patients treated for brucellosis, enteric fever
and syphilis.
Bone marrow effects
Chloramphenicol exerts a dose-related but reversible depressant
effect on the marrow of all those treated, resulting in
vacuolization of erythroid and myeloid cells, reticulocytopenia
and ferrokinetic changes indicative of decreased erythropoiesis.
Evidence of bone-marrow depression is regularly
seen if the plasma concentration exceeds 25 mg/L, and leukopenia
and thrombocytopenia may be severe. There is no
evidence that this common marrow depression is the precursor
of potentially fatal aplasia, which differs in that it is fortunately
rare, late in onset, usually irreversible and may follow
the smallest dose. Aplasia can follow systemic, oral and even
ophthalmic administration and may be potentiated by cimetidine.
Liver disease, uremia and pre-existing bone marrow
dysfunction may increase the risk. It is unusual for manifestations
to appear during treatment, and the interval between
cessation of treatment and onset of dyscrasia can be months.
A few patients survive with protracted aplasia, and myeloblastic
leukemia then often supervenes.
It is thought that the toxic agent is not chloramphenicol
itself but an as yet unidentified metabolite. Chloramphenicol
is partially metabolized to produce oxidized, reduced and
conjugated products. The toxic metabolite may be a shortlived
product of reduction of the nitro group, which damages
DNA by helix destabilization and strand breakage.
Predisposition to aplasia may be explained by genetically
determined differences in metabolism of the agent. Risk
of fatal aplastic anemia has been estimated to increase
13-fold on average treatment with 4 g of chloramphenicol.
Corresponding increases are 10-fold in patients treated with
mepacrine (quinacrine) and 4-fold in patients treated with
oxyphenbutazone.
Children
Infants given large doses may develop exceedingly high
plasma levels of the drug because of their immature conjugation
and excretion mechanisms. A life-threatening disorder
called the ‘gray baby’ syndrome, characterized
by vomiting, refusal to suck and abdominal distention followed
by circulatory collapse,
may appear when the plasma
concentration exceeds 20 mg/L. If concentrations reach
200 mg/L, the disorder can develop in older children or
even adults.
Optic neuritis has been described in children with cystic
fibrosis receiving prolonged treatment for pulmonary infection.
Most improve when the drug is discontinued, but central
visual acuity can be permanently impaired. There is some
experimental evidence that ear drops containing 5% chloramphenicol
sodium succinate can damage hearing. One study
identified an increased risk of acute leukemia following childhood
administration of chloramphenicol, particularly for
durations exceeding 10 days. | | Safety Profile | Confirmed human
carcinogen producing leukemia, aplastic
anemia, and other bone marrow changes.
Experimental tumorigenic data. Poison by
intravenous and subcutaneous routes.
Moderately toxic by ingestion and
intraperitoneal routes. Human systemic
effects by an unknown route: changes in
plasma or blood volume, unspecified liver
effects, and hemorrhaging. Experimental
teratogenic and reproductive effects. Human
mutation data reported. An antibiotic. When
heated to decomposition it emits very toxic
fumes of NOx and Cl-. See also other
chloramphenicol entries. | | Synthesis | Chloramphenicol, D-threo-2,2-dichloro-N-[|?-hydroxy-|á-(hydroxymethyl)]-n-nitropheny�lacetamide (32.6.7), was first isolated in 1947 from a culture fluid of the actinomycete
Streptomyces venezuelae; however, it is only currently produced synthetically. When using
a synthetic racemic mixture without having previously separated it into D- and L-threo
forms, it is called sintomycin. Two ways of synthesizing chloramphenicol are suggested.
The first begins with 4-nitroacetophenone, which is brominated with molecular bromine
to make |?-bromo-4-nitroacetophenone (32.6.1). This is transformed to |?-amino-
4-nitroacetophenone (32.6.2) by successive production of a quaternary salt with urotropine
and subsequent break up to an amine using hydrogen chloride. The resulting aminoketone
is acylated with acetic anhydride to make |?-acetamido-4-nitroacetophenone (32.6.3), and
the product undergoes acylmethylation with paraform aldehyde to give |á-acetamido-
|?-hydroxy-4-nitropropiophenone (32.6.4). Reducing the carbonyl group in the resulting
compound with aluminum isopropoxide in isopropyl alcohol gives D,L-threo-2-acetamido-
1-(4-nitrophenyl)-1,3-propandiol (32.6.5). The acetyl group is hydrolyzed in hydrochloric
acid to form D,L-threo-2-amino-1(4-nitrophenyl)-1,3-propandiol. The resulting racemic
mixture of amines is treated with camphor-D-sulfonic acid, and the resulting enantiomeric
salts are separated. After alkaline hydrolysis of the selected salt, the product D, (?)-threo-2-
amino-1-(4-nitrophenyl)-1,3-propandiol (32.6.6) is synthesized. Acylating the aminogroup
of this compound with the methyl ester of dichloroacetic acid gives the desired chloram�phenicol (32.6.7). 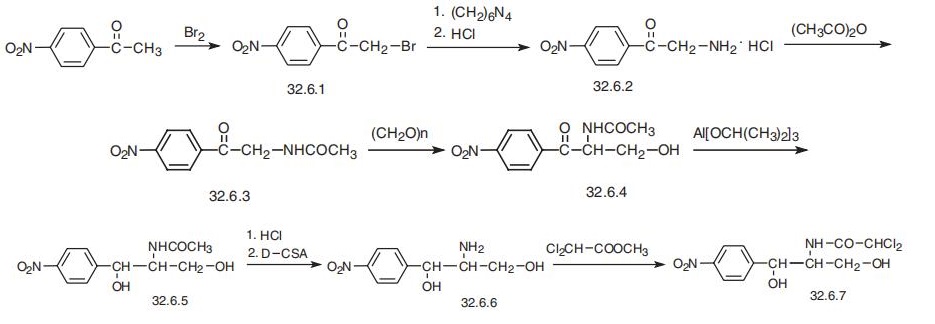
The other synthesis begins with cinnamic alcohol, which is reacted with hypobromous
acid to make 2-bromo-1-phenyl-1,3-propandiol (32.6.8), the hydroxyl group of which is
protected as a ketal by reacting it with acetone, giving 5-bromo-2,2-dimethyl-4-phenyl-
1,3-dioxane (32.6.9). Reacting the resulting bromide with ammonia gives an isomeric
mixture of D,L-threo-5-amino-2,2-dimethyl-4-phenyl-1,3-dioxane, which upon treatment
with D-tartaric acid, separation of the resulting salts, and subsequent alkaline hydrolysis
of the selected salt gives D-(?)-5-amino-2,2-dimethyl-4-phenyl-1,3-dioxane (32.6.10).
Acylating this with the methyl ester of dichloroacetic acid gives D-(?)-threo-5-dichloroac�etamido-2,2-dimethyl-4-phenyl-1,3-dioxane (32.6.11). The phenyl ring is then nitrated,
during which the 1,3-dioxane ring is cleaved off, giving dinitrate of D-(?)-threo-2-
dichloroacetamido-1-(4-nitrophenyl)-1,3-propandiol (32.6.12). Reducing the nitro group
in this compound with bivalent iron sulfate gives the desired chloramphenicol (32.6.7).
| | Potential Exposure | An antibiotic derived from streptomyces venezuelae. A potential danger to those involved in the
manufacture, formulation, and application of this antibiotic
and antifungal agent | | Veterinary Drugs and Treatments | Chloramphenicol is used for a variety of infections in small animals
and horses, particularly those caused by anaerobic bacteria. The
FDA has prohibited the use of chloramphenicol in animals used for
food production because of the human public health implications. | | Drug interactions | Potentially hazardous interactions with other drugs
Anticoagulants: effect of coumarins enhanced.
Antidiabetics: effect of sulphonylureas enhanced.
Antiepileptics: metabolism accelerated by
phenobarbital and primidone (reduced concentration
of chloramphenicol); increased concentration of
fosphenytoin and phenytoin (risk of toxicity).
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Ciclosporin: possibly increases ciclosporin
concentration.
Clopidogrel: possibly reduces antiplatelet effect.
Tacrolimus: possibly increases tacrolimus
concentration. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med?ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ?ing resuscitation mask) if breathing has stopped and CPR if598 Chloramphenicolheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | | Carcinogenicity | Chloramphenicol is reasonably anticipated to be a human carcinogen, based on limited evidence of carcinogenicity from studies in humans. | | Environmental Fate | As an antibiotic, chloramphenicol enters the target cells by
facilitated diffusion and binds reversibly to the 50S ribosomalsubunit. This prevents the interaction between peptidyl transferase
and its amino acid substrate, which results in the inhibition
of peptide bond formation. Indeed, it is an inhibitor of
protein synthesis in the bacteria and to a lesser extent, in
eukaryotic cells. Chloramphenicol can also inhibit mitochondrial
protein synthesis in mammalian cells particularly erythropoietic
cells, which are sensitive to the drug. | | Metabolic pathway | Six metabolites of chloramphenicol are identified,
among which the sulfate conjugate is characterized in
goat urine. | | Metabolism | When given orally, it is rapidly and completely absorbed but has a fairly short half-life. It is mainly excreted
in the urine in the form of its metabolites, which are a C-3 glucuronide, and, to a lesser extent, its
deamidation product and the product of dehalogenation and reduction. These metabolites are all inactive.
The aromatic nitro group also is reduced metabolically, and this product can undergo amide hydrolysis. The
reduction of the nitro group, however, does not take place efficiently in humans but, rather, primarily occurs
in the gut by the action of the normal flora. Chloramphenicol potentiates the activity of some other drugs by
inducing liver metabolism. Such agents include anticoagulant coumarins, sulfonamides, oral hypoglycemics,
and phenytoin. | | storage | Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working withChloramphenicol you should be trained on its proper handling and storage. A regulated, marked area should beestablished where this chemical is handled, used, or storedin compliance with OSHA Standard 1910.1045. Store intightly closed containers in a cool, well-ventilated area. | | Shipping | UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic
solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-
Poisonous materials, Technical Name Required. | | Purification Methods | Purify chloramphenicol by recrystallisation from H2O (solubility is 2.5mg/mL at 25o) or ethylene dichloride as needles or long plates, and by sublimation at high vacuum. It has A 1cm 298 at max 278nm, and it is slightly soluble in H2O (0.25%) and propylene glycol (1.50%) at 25o but is freely soluble in MeOH, EtOH, BuOH, EtOAc and Me2CO. [Relstock et al. J Am Chem Soc 71 2458 1949, Confroulis et al. J Am Chem Soc 71 2463 1949, Long & Troutman J Am Chem Soc 71 2469, 2473 1949, Ehrhart et al. Chem Ber 90 2088 1957, Beilstein 13 IV 2742.] | | Toxicity evaluation | In the aquatic system, chloramphenicol is not expected to
adsorb to suspended solids and sediments given by the Koc
(Soil Organic Carbon–Water Partitioning Coefficient) value
of 99.
Chloramphenicol solutions are susceptible to direct photolysis
by sunlight or high temperatures and decompose to form
hydrochloric and dichloric acid. Hydrolysis of chloramphenicol
is not anticipated under environmental conditions because
it lacks a functional group to hydrolyze. Chloramphenicol has
been reported to degrade 86.2% with a biodegradation rate of
3.3 mg COD per gram per hour using adapted activated sludge
as the inoculums. It can also be degraded by intestinal bacteria
via amidolysis to 18 observed metabolites. | | Incompatibilities | Compounds of the carboxyl group react
with all bases, both inorganic and organic (i.e., amines),
releasing substantial heat, water, and a salt that may be
harmful. Incompatible with arsenic compounds (releases
hydrogen cyanide gas), diazo compounds, dithiocarbamates,
isocyanates, mercaptans, nitrides, sulfides (releasing heat,
toxic, and possibly flammable gases), thiosulfates, and
dithionites (releasing hydrogen sulfate and oxides of sulfur). | | Waste Disposal | It is inappropriate and possibly
dangerous to the environment to dispose of expired or waste
pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or
waste pharmaceuticals may be mixed with wet cat litter or
coffee grounds, double-bagged in plastic, discard in trash.
Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the
pharmaceutical to the manufacturer for proper disposal being
careful to properly label and securely package the material.
Alternatively, the waste pharmaceutical shall be labeled,
securely packaged, and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. |
| | Chloromycetin Preparation Products And Raw materials |
|