| Company Name: |
Jinan Yuanmao Chemical Co., Ltd
|
| Tel: |
15665725027 18854103888 |
| Email: |
980803361@qq.com |
| Products Intro: |
Cas:1305-62-0
ProductName:Calcium Hydroxide;Calcium Dihydroxide;Slaked Lime;Hydrated Lime
Purity: 99.5% | Package: 25kg
|
| Company Name: |
Usha Chemicals
|
| Tel: |
08046059614 |
| Email: |
|
| Products Intro: |
Cas:1305-62-0
ProductName:Slaked Lime
|
| Company Name: |
Ishwar Enterprises
|
| Tel: |
08048250473Ext 386 |
| Email: |
|
| Products Intro: |
Cas:1305-62-0
ProductName:Slaked Lime
|
| Company Name: |
|
| Tel: |
|
| Email: |
Akhilesh.sahu@unilosa.com |
| Products Intro: |
Cas:1305-62-0
ProductName:Hydrated Lime
Purity: 99% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
|
| Company Name: |
|
| Tel: |
|
| Email: |
VAYUNANDANIMPEX@GMAIL.COM |
| Products Intro: |
Cas:1305-62-0
ProductName:Hydrated Lime
Purity: 99% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
|
- quick lime
-

- $180.00/ ton
-
2024-11-06
- CAS:1305-78-8
- Min. Order: 25ton
- Purity: 99%
- Supply Ability: 1000 tons per month
- quick lime
-

- $180.00/ ton
-
2024-08-30
- CAS:1305-78-8
- Min. Order: 25ton
- Purity: 99%
- Supply Ability: 1000 tons per month
- LIME OIL
-

- $2.00 / 1KG
-
2019-12-20
- CAS:8008-26-2
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: ask
Related articles - Introduction of Calcium hydroxide
- Calcium hydroxide is a material which has been used for a variety of purposes since its introduction into dentistry in the ear....
- Nov 23,2021
|
| Product Name: | Slaked Lime | | Synonyms: | CALCIUMHYDROXIDE,POWDER,REAGENT,ACS;CALCIUMHYDROXIDE,POWDER,USP,EP,BP,JP;CALCIUMHYDROXIDE,TECH(HYDRATEDLIM)(BULK;CALCIUMHYDROXIDE,TECHNICAL;Calcium Hydroxde;Calcium Hydroxide, Powder, Reagent;Calcium hydroxide, 98+%, extra pure;Calcium hydroxide, 95+%, for analysis ACS | | CAS: | 1305-62-0 | | MF: | CaH2O2 | | MW: | 74.09 | | EINECS: | 215-137-3 | | Product Categories: | fine chemicals;Food Additives;C-D, Puriss p.a.;Analytical Reagents for General Use;Puriss p.a.;Chemical Synthesis;Inorganic Bases;Synthetic Reagents;AlgicidesMetal and Ceramic Science;Calcium Salts;Pesticides;Pesticides&Metabolites;Salts;ACS GradeChemical Synthesis;Essential Chemicals;Routine Reagents;Inorganics | | Mol File: | 1305-62-0.mol | 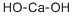 |
| | Slaked Lime Chemical Properties |
| Melting point | 580 °C | | Boiling point | 2850 °C | | bulk density | 400kg/m3 | | density | 2.24 g/mL at 25 °C (lit.) | | refractive index | 1.574 | | storage temp. | Store at +5°C to +30°C. | | solubility | 1.7g/l | | form | Solid | | pka | 12.6[at 20 ℃] | | color | White | | Odor | Odorless | | PH Range | 12.4 | | PH | 11.27(1 mM solution);12.2(10 mM solution);12.46(100 mM solution); | | Water Solubility | 1.65 g/L (20 ºC) | | Sensitive | Air Sensitive | | Merck | 14,1673 | | Solubility Product Constant (Ksp) | pKsp: 5.26 | | Exposure limits | ACGIH: TWA 5 mg/m3
OSHA: TWA 15 mg/m3; TWA 5 mg/m3
NIOSH: TWA 5 mg/m3 | | Stability: | Stable. Incompatible with strong acids. | | CAS DataBase Reference | 1305-62-0(CAS DataBase Reference) | | NIST Chemistry Reference | Calcium dihydroxide(1305-62-0) | | EPA Substance Registry System | Calcium hydroxide (1305-62-0) |
| | Slaked Lime Usage And Synthesis |
| Chemical Properties |
Slaked Lime is a caustic, inflammable, white (or white-and-beige) powder having a crystalline structure. Slaked Lime solutions can cause chemical burns. At high pH values due to a common-ion effect with the hydroxide anion, its solubility drastically decreases. Aqueous solutions of Slaked Lime are called limewater and are medium-strength bases, which react with acids and can attack some metals such as aluminium(amphoteric hydroxide dissolving at high pH), while protecting other metals, such as iron and steel, from corrosion by passivation of their surface.

| | Physical properties |
Slaked Lime is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product Ksp of 5.02×10−6 at 25 °C. At ambient temperature, Slaked Lime dissolves in water to produce an alkaline solution with a pH of about 12.5.
| | Uses |
1.Slaked Lime is used for preparing bleaching powders, hard water softeners and the disinfecting and clarifying agents of tap water , as well as in building industry;
2.Used as rubber and oil industry additives. Used in lubricants for preventing coking, sludge deposition and anti-corrosion. Used as hard water softeners, disinfectants, antacids and so on;
3. Used as analytical reagents, carbon dioxide absorbents, as well as in organic synthesis;
4. Used for making bleaching powder, hard water softener, depilatory, disinfectant, acid stop agent, astringent and various calcium salts;
5. Use for carbon dioxide examination and gas absorption. Used for leather hair removal, insecticide and water treatment.
| | Production Methods | Slaked lime is produced when water is added to calcium oxide (CaO, or quicklime). The result of this mixing of water and calcium oxide (quicklime) is an exothermic reaction known as slaking. The slaking reaction produces calcium hydroxide, (Ca(OH)2).
| | Health Hazard | Unprotected exposure to Slaked Lime, as with any strong base, can cause severe skin irritation, chemical burns, blindness, lung damage or rashes.
| | Safety |
Wear impervious protective clothing, including boots, gloves, lab coat, apron, or coveralls, to prevent skin contact.
Dust irritates the respiratory tract. Symptoms may include coughing and shortness of breath. It can cause chemical bronchitis. Ingestion may be followed by severe pain, vomiting, diarrhea, and collapse. Corrosive. Skin and eye contact may produce severe irritation and pain.
| | Structure and conformation | Slaked Lime adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH)2 (brucite structure); i.e., the cadmium iodide motif. Strong hydrogen bonds exist between the layers.
| | Incompatibilities | Incompatible with strong acids, maleic anhydride, phosphorus, nitroethane, nitromethane, nitroparaffins, and nitropropane. Calcium hydroxide can be corrosive to some metals.
|
| | Slaked Lime Preparation Products And Raw materials |
|