| Company Name: |
MedChemexpress LLC
|
| Tel: |
021-58955995 |
| Email: |
sales@medchemexpress.cn |
| Products Intro: |
Product Name:U66858
CAS:99107-52-5
Purity:400RMB/1mg Package:>98%
|
| Company Name: |
Fan De(Beijing) Biotechnology Co., Ltd.
|
| Tel: |
15911056312 |
| Email: |
liming@bio-fount.com |
| Products Intro: |
Product Name:1-Acetoxy-2-butyl-4-methoxynaphtalene
CAS:99107-52-5
Purity:97.0% Package:5mg
|
| Company Name: |
cjbscvictory
|
| Tel: |
13348960310 13348960310 |
| Email: |
3003867561@qq.com |
| Products Intro: |
Product Name:Bunaprolast
CAS:99107-52-5
Purity:98% Package:5mg;10mg;20mg;50mg;100mg;200mg
|
1-Acetoxy-2-butyl-4-methoxynaphtalene manufacturers
- Bunaprolast
-

- $1800.00 / 5mg
-
2024-11-20
- CAS:99107-52-5
- Min. Order:
- Purity: 99.9%
- Supply Ability: 10g
|
| | 1-Acetoxy-2-butyl-4-methoxynaphtalene Basic information |
| Product Name: | 1-Acetoxy-2-butyl-4-methoxynaphtalene | | Synonyms: | 1-Acetoxy-2-butyl-4-methoxynaphtalene;Bunaprolast;1-Acetoxy-2-butyl-4-methoxynaphthalene;U-66858;1-acetoxy-2-n-butyl-4-methoxynaphthalene;1-Naphthalenol, 2-butyl-4-methoxy-, 1-acetate;Bunaprolast (U66858) | | CAS: | 99107-52-5 | | MF: | C17H20O3 | | MW: | 272.3389 | | EINECS: | | | Product Categories: | | | Mol File: | 99107-52-5.mol | 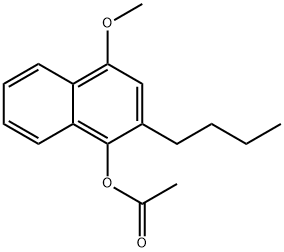 |
| | 1-Acetoxy-2-butyl-4-methoxynaphtalene Chemical Properties |
| storage temp. | Store at -20°C | | solubility | Soluble in DMSO |
| | 1-Acetoxy-2-butyl-4-methoxynaphtalene Usage And Synthesis |
| Originator | Bunaprolast,ZYF Pharm Chemical | | Uses | Anti-asthmatic. | | Manufacturing Process | Preparation of pentacarbonyl[phenyl(methoxy)carbene]chromium:
To a suspension of 22 g (0.1 mole) of chromium hexacarbonyl in ether is
slowly added phenyllithium (51 ml, 0.1 mole, cyclohexane/ether solution) via
syringe over a period of 15-20 minutes under argon at room temperature, and
the resulting deep red solution is stirred at room temperature for 1 hour. The
solvent is removed under reduced pressure (bath temperature should be
below 40°C), and the black residue is dissolved in 200 ml water. (CH3)3O·BF4
(about 15 g) is added portionwise to the solution until it becomes slightly
acidic (pH 5.5). The aqueous layer is extracted three times with 200 ml of
ether, and the combined etheral extracts are washed once with 300 ml of
saturated brine solution, once with 300 ml of saturated sodium carbonate
solution, and three times with 300 ml of saturated brine solution, dried over
anhydrous sodium sulfate and filtered. The solvent is removed using a rotary
evaporator. The deep red tarry residue is purified using flash chromatography
by a silica gel column (200 g). Elution by 10% ether in n-hexane gives a deep
red syrup, which is solidified upon cooling. Recrystallization from pet-ether at
-70°C gives 25.12 g (80.5%) of pentacarbonyl[phenyl(methoxy)carbene]
chromium as deep red crystalline. The physical properties of the product are
consistent with those described in the literature.
2-Butyl-4-methoxy-1-naphthalenol, acetate:
Reaction of pentacarbonyl[phenyl(methoxy)carbene ]chromium with 1-
hexyne:
Part A.
A mixture of the carbene complex (1.0 g, 3.2 mmole), 1-hexyne (2.6
equivalents), acetic anhydride (1.0 eq.) and triethylamine (1.0 eq.) in
tetrahydrofuran (90 ml) is heated at 65°C under an argon atmosphere for 1
hour. The solution is cooled and concentrated to give a black residue, which is
chromatographed through a silica gel (200 g) column using a flash
chromatography. Elution by10% ether in n-hexane gives 71 5 mg (82.2%) of
the title product, which solidified. Recrystallization from pet-ether gave white
crystals of 2-butyl-4-methoxy-1-naphthalenol acetate; MP: 49°C.
Part B.
Alternatively, a mixture of 2.0 g (6.4 mmole) of the carbene complex,
acetylene (2.6 eq.) and acetic acid in tetrahydrofuran is heated at 65°C for 2
hours under argon atmosphere. After cooling the reaction solution is
concentrated and the black residue is loaded on a silica gel (200 g) column for
a flash chromatography. Elution by 10% ether in n-hexane gives 895 mg
(51.4%) of the title product. | | Therapeutic Function | Anti-asthmatic | | in vivo | The IgE-mediated hypersensitivity to Ascaris antigen in reactor rhesus primates is used to assess the pharmacologic profile of Bunaprolast (U-66,858). When Bunaprolast is given by the oral route, it shows dose-related inhibition of resistance (RL) and compliance (Cdyn) changes. When Bunaprolast is given by the aerosol route, it shows dose independent inhibition. In 15 animals, aerosols (52±32 to 53±10% for RL, p=0.05 and 45±19 to 28±19% Cdyn inhibitions, p=0.05) for 5.0-0.1% aerosol. By the oral route, inhibition is seen at 1-4 h following administration. In 5 animals, oral doses of 10 and 5 mg/kg inhibit (RL by 98±2 to 78±1.5%, p=0.01 and Cdyn by 75±17 to 60.9±9.1%, p=0.05) by 10 and 5 mg/kg Bunaprolast, respectively[2]. | | IC 50 | LTB4; TXB2; 5-LO |
| | 1-Acetoxy-2-butyl-4-methoxynaphtalene Preparation Products And Raw materials |
|