- 3,5-Dimethoxytoluene
-

- $45.00 / 1kg
-
2025-03-31
- CAS:4179-19-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 tons
- 3,5-Dimethoxytoluene
-

- $50.00 / 500mg
-
2024-11-19
- CAS:4179-19-5
- Min. Order:
- Purity: 99.06%
- Supply Ability: 10g
- 3,5-Dimethoxytoluene
-

- $15.00 / 1KG
-
2021-08-12
- CAS:4179-19-5
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | 3,5-Dimethoxytoluene Basic information |
| Product Name: | 3,5-Dimethoxytoluene | | Synonyms: | 3,5-DIMETHOXYTOLUENE;3,5-DiMethoxytoluene, GC 98%;ORCINOL DIMETHYL ETHER;3,5-Dimethoxy-1-methylbenzene;5-Methylresorcinol dimethyl ether;Benzene, 1,3-dimethoxy-5-methyl-;Toluene, 3,5-dimethoxy-;1,5-Dimethoxy-3-methylbenzene | | CAS: | 4179-19-5 | | MF: | C9H12O2 | | MW: | 152.19 | | EINECS: | 224-048-9 | | Product Categories: | Building Blocks;C9;Aromatic Ethers;Ethers;Organic Building Blocks;Oxygen Compounds;Chemical Synthesis;Organic Building Blocks;Oxygen Compounds | | Mol File: | 4179-19-5.mol | 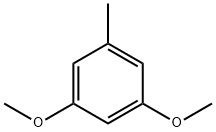 |
| | 3,5-Dimethoxytoluene Chemical Properties |
| Melting point | 61-62 C | | Boiling point | 244 °C (lit.) | | density | 1.039 g/mL at 25 °C (lit.) | | refractive index | n20/D 1.522(lit.) | | Fp | 215 °F | | storage temp. | Sealed in dry,Room Temperature | | solubility | Chloroform (Sparingly), Methanol (Sparingly) | | form | Liquid | | Specific Gravity | 1.039 | | color | Clear colorless to yellow | | Odor | Warm and sweet, nut-like, earthy-mossy odor | | BRN | 2043558 | | LogP | 2.897 (est) | | CAS DataBase Reference | 4179-19-5(CAS DataBase Reference) | | NIST Chemistry Reference | 3,5-Dimethoxytoluene(4179-19-5) | | EPA Substance Registry System | 3,5-Dimethoxytoluene (4179-19-5) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | TSCA | Yes | | HS Code | 29093090 |
| | 3,5-Dimethoxytoluene Usage And Synthesis |
| Chemical Properties | Clear colorless to yellow liquid | | Uses | 3,5-Dimethoxytoluene (DMT) may be used in the synthesis of 3,5-dimethoxybenzoic acid by oxidation and 2-methoxy-6-methyl-1,4-benzoquinone by catalytic oxidation with hydrogen peroxide (H2O2)/methyltrioxorhenium (CH3ReO3) in dimethyl carbonate (DMC). | | Uses | 3,5-Dimethoxytoluene has been suggested for
use in perfume compositions as supporting
note for Oakmoss and Vetiver, and as a novel
note in Chypre, Fougere and Oriental fragrance types. | | Definition | ChEBI: 3,5-dimethoxytoluene is a member of the class of toluenes that is toluene in which the hydrogens at positions 3 and 5 have been replaced by methoxy groups. It is the major scent compound of many rose varieties. It has a role as a fragrance and a plant metabolite. It is a member of toluenes and a member of methoxybenzenes. | | Production Methods | 3,5-Dimethoxytoluene can be produced from Orcinol in weak aqueous alkali
with Dimethylsulfate at controlled temperature. | | Synthesis Reference(s) | Organic Syntheses, Coll. Vol. 6, p. 859, 1988
The Journal of Organic Chemistry, 70, p. 3275, 2005 DOI: 10.1021/jo050075r | | General Description | 3,5-Dimethoxytoluene (DMT) is a methoxylated phenolic derivative. It is reported to be one of the main constituent of the floral volatiles in different rose varieties. It has been biosynthesized from orcinol by two successive methylation catalyzed by O-methyltransferases (OMTs). The features of its aerobic oxidation with metal/bromide catalysts have been investigated. | | Synthesis | A solution of dry orcinol (14.2 g,0.115 mole) in dry anhydrous acetone(150 ml) containing anhydrous potassium carbonate (45 g) is refluxed withdimethyl sulphate (23.6 ml,31.46 g, 0.25 mole) for 4 hr. The dimethylsulphate is added in small lots of 5 ml. After refluxing, the solution isfiltered and the inorganic residue washed with hot acetone (2 x 25 ml).The combined acetone solution is distilled and the residue macerated withcrushed ice. It is extracted with ether, the ether extract dried (anhydroussodium sulphate) and distilled. Yield 14.2 g (81.6%).B.p.102° (8 mm)
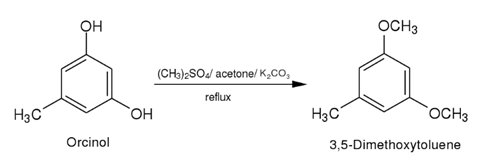 |
| | 3,5-Dimethoxytoluene Preparation Products And Raw materials |
|