- MERCUROUS SULFATE
-

- $15.00 / 1KG
-
2021-08-12
- CAS:7783-36-0
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | MERCUROUS SULFATE Basic information |
| Product Name: | MERCUROUS SULFATE | | Synonyms: | MERCURY(I) SULFATE;MERCURY(+1)SULFATE;MERCUROUS SULFATE;MERCURY(I) SULFATE EXTRA PURE 98+%;MERCUROUSSULFATE,POWDER,REAGENT;MERCUROUSSULFATE,POWDER,TECHNICAL;Quecksilber(I)-sulfat;Sulfuric acid, mercury salt | | CAS: | 7783-36-0 | | MF: | Hg2O4S | | MW: | 497.24 | | EINECS: | 231-993-0 | | Product Categories: | Inorganics | | Mol File: | 7783-36-0.mol | 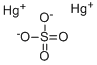 |
| | MERCUROUS SULFATE Chemical Properties |
| Melting point | decomposes [AES93] | | density | 7.56 g/mL at 25 °C(lit.) | | solubility | soluble in dilute HNO
3 | | form | Powder | | color | White, pale yellow or beige | | Water Solubility | 0.05g/100g H2O [KIR81]; soluble dilute HNO3 [MER06] | | Merck | 13,5924 | | Solubility Product Constant (Ksp) | pKsp: 6.19 | | CAS DataBase Reference | 7783-36-0(CAS DataBase Reference) | | EPA Substance Registry System | Sulfuric acid, dimercury(1+) salt (7783-36-0) |
| | MERCUROUS SULFATE Usage And Synthesis |
| Description | Mercury(I) sulfate, commonly called mercurous sulfate, Hg2SO4, is a metallic compound that forms a white, pale yellow
or beige powder. It is a metallic salt of sulfuric acid produced by replacing both hydrogen atoms with mercury(I). It is
very toxic; it can be fatal if inhaled, ingested, or absorbed by skin. The crystal structure of mercurous sulfate is made up
of Hg2
21 dumbbells and SO4
22 anions as main building units. The Hg2
21 dumbbell is surrounded by 4 oxygen atoms with
Hg?O distance between 2.23 and 2.93 ? , while the Hg-Hg distance is about 2.50 ? . Mercury(I) sulfate has the mercury
atoms arranged in doublets with a bond distance of 2.50 ? . The metal atom doublets are oriented parallel to the a-axis
in a unit cell. Mercury doublets form part of infinite chain SO4-Hg-Hg-SO4-Hg-Hg-... The Hg-Hg-O bond angle is
165° ± 1° . The chain crosses the unit cell diagonally. The mercury sulfate structure is held together by weak Hg-O interactions. The SO4 does not act as a single anion, but rather coordinated to the mercury metal. | | Chemical Properties | White, pale yellow or beige powder | | Uses | For making electric batteries; with zinc sulfate in the standard Clark cell and with cadmium sulfate in the standard Weston cell. | | General Description | White to yellow crystalline powder. Soluble in hot sulfuric acid, dilute nitric acid. Highly toxic. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | MERCUROUS SULFATE is incompatible with the following: Acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper . | | Hazard | Highly toxic. | | Synthesis | One method to produce mercury(I) sulfate is to react the acidic solution of mercury(I) nitrate with a concentrated sulfuric acid solution.
Hg2 (NO3)2 + H2SO4→Hg2SO4 + 2HNO3
It can also be formed by reacting an excess of mercury with concentrated sulfuric acid.
2Hg + 2H2SO4→Hg2SO4 + 2H2O + SO2 | | Purification Methods | The white-yellow powder is recrystallised from dilute H2SO4., dried in a vacuum under N2, and stored in the dark. Its solubility in H2O is 0.6% at 25o. It is hydrolysed by excessive washing with H2O to form the greenish-yellow basic salt Hg2SO4.Hg2O.H2O. |
| | MERCUROUS SULFATE Preparation Products And Raw materials |
|