- LY2835219
-

- $7.00 / 1KG
-
2019-12-31
- CAS:1231930-82-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG
|
| Product Name: | Abemaciclib mesylate (LY2835219) | | Synonyms: | 2-Pyrimidinamine, N-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl]-, methanesulfonate (1:1);N-[5-[(4-Ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl]-2-pyrimidinamine methanesulfonate (1:1) LY2835219;LY2835219 mesylate;Bemaciclib(salt);N-[5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl]-5-fluoro-4-(7-fluoro-2-methyl-3-propan-2-ylbenzimidazol-5-yl)pyrimidin-2-amine,methanesulfonic acid;N-(5-((4-Ethylpiperazin-1-yl)methyl)pyridin-2-yl)-5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-amine methanesulfonate;N-[5-[(4-Ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methyle;LY2835219 Ms salt, Abemaciclib Ms salt | | CAS: | 1231930-82-7 | | MF: | C28H36F2N8O3S | | MW: | 602.71 | | EINECS: | | | Product Categories: | Inhibitors | | Mol File: | 1231930-82-7.mol | 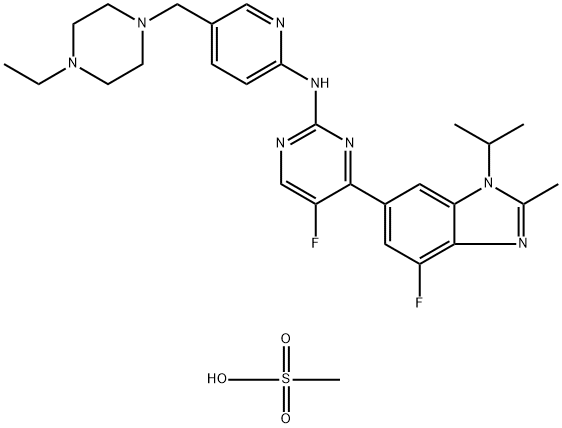 |
| | Abemaciclib mesylate (LY2835219) Chemical Properties |
| storage temp. | -20° | | solubility | Soluble in DMSO (up to at least 25 mg/ml) or in Water (up to at least 25 mg/ml). | | form | solid | | color | Off-white | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 1 month. | | CAS DataBase Reference | 1231930-82-7 |
| | Abemaciclib mesylate (LY2835219) Usage And Synthesis |
| Mechanism of action | Many human tumors acquire alterations, which can lead to the activation of cyclin-dependent kinases (CDKs)—CDK4 and CDK6. These alterations include mutations that directly activate CDK4 and CDK6, gene amplifications, which increase expression of various protein activators such as cyclin D, as well as genetic losses, which reduce expression of protein inhibitors such as p16.
Abemaciclib(previously known as LY2835219) specifically inhibits CDK4 and 6, thereby inhibiting retinoblastoma (Rb) protein phosphorylation in early G1. Inhibition of Rb phosphorylation prevents CDK-mediated G1-S phase transition, thereby arresting the cell cycle in the G1 phase, suppressing DNA synthesis and inhibiting cancer cell growth. Overexpression of the serine/threonine kinases CDK4/6, as seen in certain types of cancer, causes cell cycle deregulation.
| | Current clinical trials | Abemaciclib(LY2835219) is being investigated in clinical trials in patients with breast cancer, non-small cell lung cancer, and pancreatic cancer, including a combination clinical trial in immuno-oncology.
As of early 2016 Abemaciclib is involved in 3 Phase III clinical trials:
The JUNIPER Study is comparing Abemaciclib against Erlotinib in patients with stage IV Non-small-cell lung carcinoma.
The MONARCH 2 study is investigating the effectiveness of Abemaciclib in combination with Fulvestrant for women with breast cancer. It is due to end in Feb 2017.
The MONARCH 3 study is investigating the effectiveness of Abemaciclib, plus either anastrozole or letrozole, as a first-line treatment for women with breast cancer. The trail is expected to end in June 2017. | | Study in vivo | The dose percentage of LY2835219-MsOH treating brain was 0.5-3.9%. LY2835219-MsOH could treat subcutaneous and intracranial glioma models (U87MG), inhibiting tumor growth, and the effect is dose-dependent, whether alone or in combination with mozolomide. | | References | http://www.lillyoncologypipeline.com/molecule/cdk-4-and-cdk-6-inhibitor/overview
https://en.wikipedia.org/wiki/Abemaciclib
https://www.cancer.gov/publications/dictionaries/cancer-drug?cdrid=706364 | | Description | Abemaciclib (1231930-82-7) is a potent and selective CDK4/6 inhibitor (IC50?= 2 nM and 10 nM respectively).1?It caused G1 cell cycle arrest in colo-205 colorectal cells, MDA-MB-361 breast cancer cells, and MV4-11 AML cells. Abemaciclib was also active in several human tumor xenograft models. It displayed efficacy in patients with various solid tumors including breast cancer, non-small cell lung cancer. Glioblastoma, melanoma, colorectal cancer, and hormone receptor-positive breast cancer.2?Abemaciclib induced a T cell inflamed tumor microenvironment and enhanced the efficacy of PD-L1 checkpoint blockade in MCF-7 breast cancer cells.3?FDA approved for the treatment of advanced breast cancers. | | Uses | N-[5-[(4-Ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5-fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl]-2-pyrimidinamine is an CDK4/6 protein kinase inhibitor with potential antitumor effects. | | Uses | LY2835219 is an orally-bioavailable dual inhibitor of cyclin-dependent kinase 4 (CDK4) and CDK6 (IC50s = 2 and 10 nM, respectively). Through this mechanism, it blocks phosphorylation of retinoblastoma protein, resulting in arrest of cell cycling in the G1 phase. LY2835219 has antitumor action against xenografts when used alone or in combination with other chemotherapeutic compounds.[Cayman Chemical] | | Enzyme inhibitor | This oral cell cycle inhibitor (FWfree-base = 506.61 g/mol; FWmesylate-salt = 602.70 g/mol; CAS 1231930-82-7 (mesylate salt)), also known as LY2835219 and N-[5-[(4-ethyl-1-piperazinyl)methyl]-2-pyridinyl]-5- fluoro-4-[4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl]-2- pyrimidinamine, targets the cyclin-dependent kinase CDK4, or cyclin D1 (IC50 = 2 nM) and CDK6, or cyclin D3 (IC50 = 6 nM), inhibiting retinoblastoma (Rb) protein phosphorylation in early G1, thereby arresting the cell cycle in the G1, suppressing DNA synthesis, and inhibiting cancer cell growth. LY2835219 inhibits activation of AKT and ERK, but not mTOR. | | References | 1) Gelbert?et al.?(2014),?Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine; Invest. New Drugs,?32?825
2) Patnail?et al.?(2016),?Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-small Cell Lung Cancer, and Other Solid Tumors; Cancer Discov.,?6?740
3) Schaer?et al. (2018),?The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade; Cell Rep.,?22?2978 |
| | Abemaciclib mesylate (LY2835219) Preparation Products And Raw materials |
|