DMT-2'-Fluoro-dG(Ib) Phosphoramidite manufacturers
|
| | DMT-2'-Fluoro-dG(Ib) Phosphoramidite Basic information |
| Product Name: | DMT-2'-Fluoro-dG(Ib) Phosphoramidite | | Synonyms: | 5'-O-[Bis(4-methoxyphenyl)phenylmethyl]-2'-deoxy-2'-fluoro-N-(2-methyl-1-oxopropyl)guanosine 3'-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite];DMT-2FLUORO-DG(IB) AMIDITE;DMT-2'-F-dG(iBu)-CE Phosphoramidite;2'-F-dG(iBu)-CE Phosphoramidite;5'-O-DMT-2'-fluoro-N2-isobutyryl-2'-Deoxy-guanosine 3'-CE phosphoramidite;N-[9-[(2R,3R,4R,5R)-5-[[bis(4-methoxyphenyl)-phenylmethoxy]methyl]-4-[2-cyanoethoxy-[di(propan-2-yl)amino]phosphanyl]oxy-3-fluorooxolan-2-yl]-6-oxo-3H-purin-2-yl]-2-methylpropanamide;ibu-2'-F-dG Phosphoramidite;2'-fluoro-N2-isobutyryl-5'-O-(4, 4'-dimethoxytrityl)-2'-deoxyguanosine-3'-CE-Phosphoramidite | | CAS: | 144089-97-4 | | MF: | C44H53FN7O8P | | MW: | 857.91 | | EINECS: | | | Product Categories: | Amidite | | Mol File: | 144089-97-4.mol | 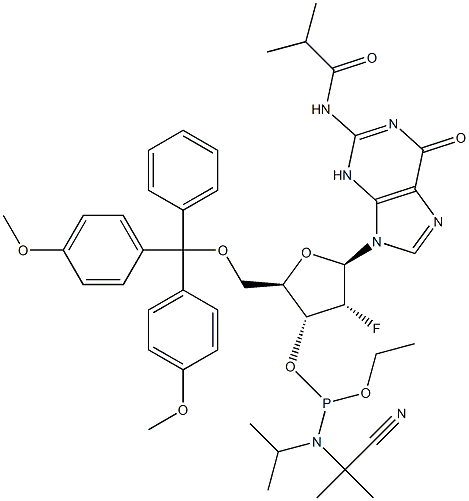 |
| | DMT-2'-Fluoro-dG(Ib) Phosphoramidite Chemical Properties |
| storage temp. | -20°C | | solubility | DMSO : 100 mg/mL (116.56 mM) | | form | powder | | pka | 9.16±0.20(Predicted) | | color | white to off-white | | biological source | non-animal source (no BSE/TSE risk) | | InChIKey | CFFUQMXILRDKMM-QPYLYBSMNA-N | | SMILES | C(NC1=NC(=O)C2=C(N1)N([C@H]1[C@H](F)[C@H](OP(OCC)N(C(C)C)C(C#N)(C)C)[C@@H](COC(C3=CC=C(OC)C=C3)(C3=CC=C(OC)C=C3)C3=CC=CC=C3)O1)C=N2)(=O)C(C)C |&1:10,11,13,28,r| |
| | DMT-2'-Fluoro-dG(Ib) Phosphoramidite Usage And Synthesis |
| Description | DMT-2'-Fluoro-dG(Ib) Phosphoramidite is a 2′Fluoro phosphoramidite that is used to synthesize oligonucleotides that are more thermally stable and provide increased nuclease resistance.
| | Uses | DMT-2''Fluoro-DG(IB) amidite (CAS# 144089-97-4) is a nucleoside used in the preparation of cyclic dinucleotide compounds, and in the preparation of 4''-C-methoxy-2''-deoxy-2''-fluoro modified ribonucleotides, which improve metabolic stability and elicit efficient RNAi-mediated gene silencing. | | Definition |
The key features of DMT-2'-Fluoro-dG(Ib) Phosphoramidite include, Can be employed together with DNA or RNA phosphoramidites Recommended deprotection conditions are 8 hours at 55 °C using concentrated ammonia solution, or with AMA for 10 minutes at 65 °C Synthesis of 2′Fluoro oligonucleotides is similar to standard DNA synthesis but requires an elongated coupling time (recommended is 3 minutes compared to 90 seconds for DNA monomers) Consistent lot-to-lot high purity and performance Manufactured under a certified ISO 9001 quality system.
|
| | DMT-2'-Fluoro-dG(Ib) Phosphoramidite Preparation Products And Raw materials |
|