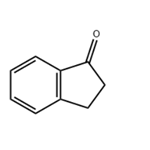
- 1-Indanone
-

- $0.00 / 1KG
-
2025-04-10
- CAS:83-33-0
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- 1-Indanone
-

- $0.00 / 1kg
-
2025-04-10
- CAS:83-33-0
- Min. Order: 1kg
- Purity: 98
- Supply Ability: 1000
- 1-Indanone
-
- $0.00 / 1KG
-
2025-04-04
- CAS:83-33-0
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1Ton
|
| | 1-Indanone Basic information |
| | 1-Indanone Chemical Properties |
| Melting point | 38-40 °C(lit.) | | Boiling point | 243-245 °C(lit.) | | density | 1.103 g/mL at 25 °C(lit.) | | refractive index | 1.5610 (estimate) | | Fp | 233 °F | | storage temp. | Store below +30°C. | | solubility | 6.5g/l | | form | Crystalline Mass | | color | Light yellow to brown | | Odor | Rather weak, woody and somewhat medicinal odor with an incense-like undertone. | | biological source | mouse | | Odor Threshold | 0.0088ppm | | Water Solubility | 6.5 g/L (20 C) | | BRN | 507957 | | Stability: | Light sensitive | | InChIKey | QNXSIUBBGPHDDE-UHFFFAOYSA-N | | CAS DataBase Reference | 83-33-0(CAS DataBase Reference) | | NIST Chemistry Reference | 1H-Inden-1-one, 2,3-dihydro-(83-33-0) | | EPA Substance Registry System | 1H-Inden-1-one, 2,3-dihydro- (83-33-0) |
| Hazard Codes | Xn,Xi | | Risk Statements | 22-36/37/38 | | Safety Statements | 22-24/25-36-26 | | WGK Germany | 3 | | Autoignition Temperature | 525 °C | | Hazard Note | Irritant | | TSCA | Yes | | HS Code | 29143900 |
| | 1-Indanone Usage And Synthesis |
| Chemical Properties | Pale yellow liquid | | Uses | 1-Indanone is an oxidation product of indan, a component of fuels, solvents and varnishes. 1-Indanone is also a metabolite of Thalidomide that has been shown to inhibit the attachment of tumor cells to concanavalin A coated plastic surfaces. | | Uses | 1-Indanone is an oxidation product of Indan, a component of fuels, solvents, and varnishes. 1-Indanone is also a metabolite of Thalidomide that has been shown to inhibit the attachment of tumor cells to concanavalin A coated plastic surfaces. | | Definition | ChEBI: An indanone that consists of 2,3-dihydro-1H-indene substituted by an oxo group at position 1. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 54, p. 1144, 1989 DOI: 10.1021/jo00266a028
Journal of the American Chemical Society, 99, p. 4822, 1977 DOI: 10.1021/ja00456a048
Tetrahedron Letters, 27, p. 3139, 1986 DOI: 10.1016/S0040-4039(00)84736-X | | Biological Activity | Indoleamine 2,3 dioxygenase (IDO) 1 plays an important role in the metabolism of tryptophan and in immune regulation. Removal of IDO1 results in attenuated contact hypersensitivity (CHS) responses. | | Synthesis | 1-Indanone is produced by cyclization of be{a-Phenylpropionyl chloride in Benzene. |
| | 1-Indanone Preparation Products And Raw materials |
|