- Sudan II
-

- $56.00 / 25mg
-
2024-11-19
- CAS:3118-97-6
- Min. Order:
- Purity: 98.45%
- Supply Ability: 10g
- SUDAN II
-
- $1.00 / 1KG
-
2024-08-11
- CAS:3118-97-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T
- Sudan II
-
- $0.00 / 20mg
-
2023-02-24
- CAS:3118-97-6
- Min. Order: 5mg
- Purity: ≥90%(HPLC)
- Supply Ability: 10 g
|
| | SUDAN II Basic information |
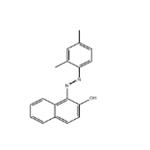
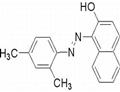
| Product Name: | SUDAN II | | Synonyms: | Sudan scarlet 6g;Sudan X;sudanorangerpa;sudanscarlet6g;Waxakol vermilion L;waxakolvermilion;waxakolvermilionl;1-[(E)-(2,4-Dimethylphenyl)diazenyl]-2-naphthol | | CAS: | 3118-97-6 | | MF: | C18H16N2O | | MW: | 276.33 | | EINECS: | 221-490-4 | | Product Categories: | Organics | | Mol File: | 3118-97-6.mol | 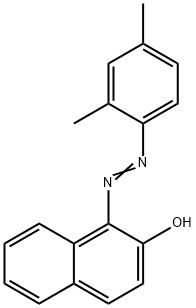 |
| | SUDAN II Chemical Properties |
| Melting point | 156-158 °C(lit.) | | Boiling point | 419.24°C (rough estimate) | | density | 1.1318 (rough estimate) | | vapor pressure | 0Pa at 25℃ | | refractive index | 1.5800 (estimate) | | storage temp. | Hygroscopic, Refrigerator, Under inert atmosphere | | solubility | Chloroform (Slightly), DMSO (Slightly, Heated, Sonicated), Ethyl Acetate (Slight | | Colour Index | 12140 | | pka | 13.52±0.50(Predicted) | | form | Powder | | color | Red to orange-brownish | | Water Solubility | 54.45μg/L at 25℃ | | λmax | 493 nm
604 nm (2nd) | | ε(extinction coefficient) | ≥14000 at 491-497nm in methanol at 0.005g/L
≥9000 at 419-425nm in methanol at 0.005g/L | | Stability: | Hygroscopic | | LogP | 6.6 | | CAS DataBase Reference | 3118-97-6 | | IARC | 3 (Vol. 8, Sup 7) 1987 | | EPA Substance Registry System | C.I. Solvent Orange 7 (3118-97-6) |
| | SUDAN II Usage And Synthesis |
| Chemical Properties | Red to orange-brownish powder | | Uses | Sudan II is a fat soluble red synthetic azo dye. Sudan II is reduced to potentially carcinogenic aromatic amines by human intestinal bacteria. Dyes and metabolites, Environmental Testing. | | Preparation | mixed N,N-dimethylaniline diazotization, and Naphthalen-2-ol coupling. | | Definition | ChEBI: Sudan II is a member of azobenzenes. | | General Description | Red crystals. Insoluble in water. | | Air & Water Reactions | Azo dyes can be explosive when suspended in air at specific concentrations. Insoluble in water. | | Reactivity Profile | SUDAN II is an azo compound. Toxic gases are formed by mixing azo compounds with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. | | Flammability and Explosibility | Not classified | | Biological Activity | Sudan II is a fat-soluble synthetic colorant and it belongs to the group of azo compounds. It is classified by the international agency for research on cancer (IARC) as category 3 carcinogens because it has an ability to cause certain forms of liver and bladder cancer in animals. | | Safety Profile | Questionable
carcinogen with experimental carcinogenic
data. Mutation data reported. When heated
to decomposition it emits toxic fumes of
NOx. | | Properties and Applications | red orange. Melting point 166 ℃ (from the glacial acetic acid crystallization out). Soluble in ethanol, acetone and benzene as the red orange. In concentrated sulfuric acid for cherry red, dilution still for cherry red, and then into the orange and red brown with precipitation; In concentrated nitric acid for brilliant orange solution, and then gradually become dark; In 10% of sodium hydroxide solution insoluble, for yellow sanguine. Dye alcohol solution to join concentrated hydrochloric acid for orange solution; Add sodium hydroxide to become red.
|
Standard
|
Light Fastness
|
Heat-resistant(℃)
|
water
|
Sodium Carbonate(5%)
|
Hydrochloric acid(5%)
|
|
Melting point
|
Stable
|
|
ISO
|
Good
|
166
|
100
|
Insoluble
|
Well
|
Well
|
|
| | SUDAN II Preparation Products And Raw materials |
|