Ropsacitinib manufacturers
- Ropsacitinib
-

- $243.00 / 1mg
-
2024-11-19
- CAS:2127109-84-4
- Min. Order:
- Purity: 99.85%
- Supply Ability: 10g
- Ropsacitinib
-

- $243.00 / 1mg
-
2024-11-19
- CAS:2127109-84-4
- Min. Order:
- Purity: 99.85%
- Supply Ability: 10g
|
| | Ropsacitinib Basic information |
| Product Name: | Ropsacitinib | | Synonyms: | (1r,3r)-3-(cyanomethyl)-3-(4-(6-(1-methyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrazin-4-yl)-1H-pyrazol-1-yl)cyclobutanecarbonitrile;PF-06826647;Tyk2-IN-8;Cyclobutaneacetonitrile, 3-cyano-1-[4-[6-(1-methyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrazin-4-yl]-1H-pyrazol-1-yl]-, trans-;PF-06826647( Tyk2-IN-8);Ropsacitinib (PF-06826647;trans-3-(Cyanomethyl)-3-(4-(6-(1-methyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrazin-4-yl)-1H-pyrazol-1-yl)cyclobutanecarbonitrile;Ropsacitinib | | CAS: | 2127109-84-4 | | MF: | C20H17N9 | | MW: | 383.41 | | EINECS: | 200-011-8 | | Product Categories: | | | Mol File: | 2127109-84-4.mol | 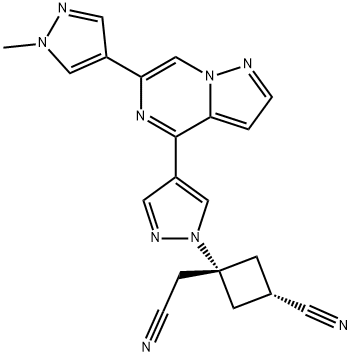 |
| | Ropsacitinib Chemical Properties |
| density | 1.46±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | DMSO: 16.67 mg/mL (43.48 mM) | | pka | 0.98±0.19(Predicted) |
| | Ropsacitinib Usage And Synthesis |
| Uses | trans-3-Cyano-1-[4-[6-(1-methyl-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrazin-4-yl]-1H-pyrazol-1-yl]cyclobutaneacetonitrile is a selective inhibitor of Tyrosine Kinase 2 (TYK2). | | Biological Activity |
The discovery of ropsacitinib, a dual JAK1/TYK2 inhibitor, was guided by previous research and molecular modeling on brepocitinib that revealed distinct hinge binding to the main chain atoms of Val981. ropsacitinib exhibited high JAK1 and JAK3 selectivity (>160-fold) and modest JAK2 selectivity (12-fold).
| | Pharmacokinetics | Ropsacitinib is neutral at physiological pH with a pKa of < 1.7. The crystalline material showed very poor thermodynamic solubility (0.3 μg/mL at pH 7.4), and the lack of a basic group precluded salt formation to improve solubility. The solubility issue was mitigated through a spray-dried dispersion (SDD) formulation, which increased experimental intestinal solubility to approximately 160?180 μM (60?70 μg/mL). Ropsacitinib displayed high passive membrane permeability in MDCK-LE cells (mean Papp = 16.7 × 10?6 cm/s) but is a substrate for MDR1 (efflux ratio > 6) and BCRP (efflux ratio > 2) efflux transport. Following oral administration of the crystalline (non SDD) material to rats at 3 and 30 mpk, ropsacitinib showed an oral bioavailability of 15% and 8% respectively. The SDD formulation increased oral bioavailability to 58% and 63% at 3 and 30 mpk, respectively. The oral bioavailability in humans was expected to be dose-dependent due to poor solubility. In healthy volunteers, the terminal elimination half-life of ropsacitinib in a single ascending dose (SAD) study is also dose-dependent: for example, approximately 6 hours at 10 mg dose and 15 hours at 400 mg dose. Ropsacitinib underwent hepatic clearance in humans primarily through the CYP450-mediated (via CYP1A2, CYP2D6, and CYP3A) metabolism, resulting in N-demethylation of the pyrazole (6), hydroxylation of the pyrazole (5), and addition of oxygen (e.g., 4) and loss of the nitrile group (e.g., 7). |
| | Ropsacitinib Preparation Products And Raw materials |
|