perhexiline manufacturers
- Perhexiline USP/EP/BP
-

- $1.10 / 1g
-
2021-08-07
- CAS:
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
|
| | perhexiline Basic information |
| Product Name: | perhexiline | | Synonyms: | perhexilene;(2R)-2-(2,2-Dicyclohexylethyl)piperidine;(R)-2-(2,2-Dicyclohexylethyl)piperidine;(2S)-2-(2,2-Dicyclohexylethyl)piperidine;perhexiline;(S)-2-(2,2-Dicyclohexylethyl)piperidine;Perhexilline;Perhexiline (Mixture of Diastereomers) | | CAS: | 6621-47-2 | | MF: | C19H35N | | MW: | 277.49 | | EINECS: | 229-569-5 | | Product Categories: | | | Mol File: | 6621-47-2.mol | 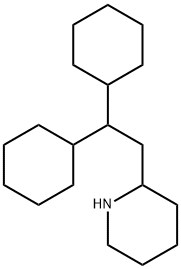 |
| | perhexiline Chemical Properties |
| Boiling point | 340.0±10.0 °C(Predicted) | | density | 0.926±0.06 g/cm3(Predicted) | | pka | pKa 10.36 ± 0.06(40% EtOH,t =25,) (Uncertain) |
| | perhexiline Usage And Synthesis |
| Originator | Pexid, Merrell-Tourade ,France ,1973 | | Uses | Vasodilator (coronary). | | Definition | ChEBI: Perhexiline is a member of piperidines. It has a role as a cardiovascular drug. | | Manufacturing Process | 1,1-Dicyclohexyl-2-(2'-pyridyl)ethanol hydrochloride (5 grams) was dehydrated by heating with 25 ml of concentrated hydrochloric acid at steam bath temperature for 10 minutes. 70 ml of water were added to the reaction mixture to give the crystalline hydrochloride salt. The product, 1,1dicyclohexyl-2-(2'-pyridyl)ethylene hydrochloride, was recrystallized from methanol-ethyl acetate to yield a white solid melting at 150°-151.5°C.
1,1-Dicyclohexyl-2-(2'-pyridyl)ethylene hydrochloride (15 grams) in 150 ml of ethanol was hydrogenated in the presence of platinum oxide at about 60 pounds per square inch of hydrogen pressure. The product, 1,1-dicyclohexyl2-(2'-piperidyl)ethane hydrochloride, crystallized from a mixture of methanol and methyl ethyl ketone as a white solid melting at 243° to 245.5°C.
The hydrochloride salt was neutralized with 10% sodium hydroxide solution and the free base so produced was dissolved in ether. The ether solution was dried over anhydrous magnesium sulfate. Addition of an excess of maleic acid in methanol to the solution yielded the acid maleate salt which melted at 188.5°-191°C.
The starting material was obtained by reacting ethyl formate with cyclohexylmagnesium bromide to give dicyclohexylcarbinol. That is oxidized to dicyclohexylketone and then reacted with α-picoline. | | Brand name | Pexid (Marion Merrell Dow). | | Therapeutic Function | Coronary vasodilator | | Enzyme inhibitor | This calcium-blocking vasodilator (FW = 277.49 g/mol; CAS 6621-47-2; typically supplied as the maleate or HCl salt), also known as Pexid? and 2- (2,2-dicyclohexylethyl)piperidine, is used to treat angina. It does so by targetting carnitine palmitoyltransferase-1 (CPT-1), an enzyme that controls the access of long chain fatty acids to the mitochondrial site of b-oxidation, showing concentration-dependent inhibition in vitro, using rat cardiac mitochondria, IC50 = 77 μM, and hepatic mitochondria, IC50 = 148 μM. Amiodarone, another drug with anti-anginal properties, likewise inhibits cardiac CPT-1 (IC50 = 228 μM). The rank order of potency for inhibition was malonyl-CoA > 4-hydroxyphenylglyoxylate = perhexiline > amiodarone = monohydroxy-perhexiline. Inhibition was competitive with respect to palmitoyl-CoA but non-competitive inhibition with respect to carnitine. This shifts myocardial metabolism from fatty acid to glucose utilisation which results in increased ATP production for the same O2 consumption and consequently increases myocardial efficiency. (See also Meldonium) Other Target(s): CYP2D6; glutathione S-transferase; Mg2+-ATPase; NADH dehydrogenase; Na+ /K+ -exchanging ATPase; b-oxidation; oxidative phosphorylation; and succinate dehydrogenase. |
| | perhexiline Preparation Products And Raw materials |
|