| Company Name: |
Shenzhen Botel Biotechnology Co. Ltd.
|
| Tel: |
13316949107 13316968096 |
| Email: |
1979313431@qq.com |
| Products Intro: |
Product Name:Isavuconazole Impurity 39
Purity:98% HPLC Package:10mg;25mg;50mg
|
| Company Name: |
Molcoo Chemicals Inc.
|
| Tel: |
17386083646 |
| Email: |
2853567991@qq.com |
| Products Intro: |
Product Name:Isavuconazole Impurity 39
CAS:207224-73-5
Purity:95% Package:25mg;30mg;50mg
|
Isavuconazole Impurity 39 manufacturers
|
| | Isavuconazole Impurity 39 Basic information |
| Product Name: | Isavuconazole Impurity 39 | | Synonyms: | Isavuconazole Impurity 39;1H-1,2,4-Triazole-1-ethanol, α-(2,4-difluorophenyl)-α-[(1R)-1-(4-phenyl-2-thiazolyl)ethyl]-, (αR)- | | CAS: | 207224-73-5 | | MF: | C21H18F2N4OS | | MW: | 412.46 | | EINECS: | | | Product Categories: | | | Mol File: | 207224-73-5.mol | 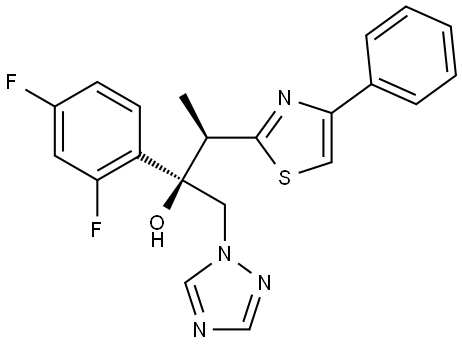 |
| | Isavuconazole Impurity 39 Chemical Properties |
| Boiling point | 622.9±65.0 °C(predicted) | | density | 1.35±0.1 g/cm3(Temp: 20 °C; Press: 760 Torr)(predicted) | | pka | 11.66±0.29(predicted) |
| | Isavuconazole Impurity 39 Usage And Synthesis |
| | Isavuconazole Impurity 39 Preparation Products And Raw materials |
|