|
|
| | Nonafluorohexyltriethoxysilane Basic information |
| Product Name: | Nonafluorohexyltriethoxysilane | | Synonyms: | NONAFLUOROHEXYLTRIETHOXYSILANE;Triethoxy(1H,1H,2H,2H-nonafluorohexyl)silane;1H,1H,2H,2H-Nonafluorohexyltriethoxysilane
Triethoxy(1H,1H,2H,2H-perfluorohexyl)silane;Triethoxy(1H,1H,2H,2H-perfluorohexyl)silane;Triethoxy(1H,1H,2H,2H-nonafluorohexyl)silane;1H,1H,2H,2H-Nonafluorohexyltriethoxysilane;1H,1H,2H,2H-PERFLUOROHEXYLTRIETHOXYSILANE;Silane,triethoxy(3,3,4,4,5,5,6,6,6-nonafluorohexyl)- | | CAS: | 102390-98-7 | | MF: | C12H19F9O3Si | | MW: | 410.35 | | EINECS: | | | Product Categories: | | | Mol File: | 102390-98-7.mol | 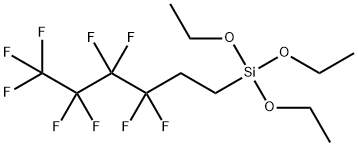 |
| | Nonafluorohexyltriethoxysilane Chemical Properties |
| Boiling point | 241.5±40.0 °C(Predicted) | | density | 1.240±0.06 g/cm3(Predicted) | | refractive index | 1.3470 to 1.3510 | | storage temp. | Inert atmosphere,Room Temperature | | form | clear liquid | | color | Colorless to Almost colorless | | Specific Gravity | 1.201 | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | EPA Substance Registry System | Triethoxy(1,1,2,2,3,3,6,6,6-nonafluorohexyl)silane (102390-98-7) |
| TSCA | Yes | | HS Code | 2931.90.9010 |
| | Nonafluorohexyltriethoxysilane Usage And Synthesis |
| Chemical Properties | Colorless transparent liquid. | | Uses | Nonafluorohexyltriethoxysilane is an organic silane, which can be used as an organic reagent. | | Application | Main applications of Nonafluorohexyltriethoxysilane(1H,1H,2H,2H-Perfluorohexyltriethoxysilane)
1) Used as a coupling agent to increase the bonding strength of the fluorine-containing resin and the substrate.
2) Used to prepare water-proof, oil-proof and anti-fouling treatment agents, and it can be used in the protection of water-proof, anti-fouling and weathering of cultural relics, masonry, metal, wood, etc.
3) Used as a finishing agent to reduce the water holding capacity of natural fiber (wool, cotton, leather, etc.) products, and to improve surface water repellency and stain resistance.
4) Used as a water-repellent and antifouling treatment on the surface of glass.
| | Production Methods | Nonafluorobutyl iodide (NFBI) and triethoxyvinylsilane (TEOVS) were fed into a three-necked flask equipped with a reflux condenser at a molar ratio of 1: 1.1 and then heated to 60 ℃ with stirring. When the internal temperature of the flask was sufficiently heated, azobisbutylnitrile (AIBN), a thermal decomposition radical initiator, was dissolved in an ether-based solvent. Then 0.5 ml/sec was dropped slowly into the stirred flask. After that, when the dropwise addition was completed, the external heating temperature was again set to 60 ℃ and stirred for 6 hours. After that, the internal temperature of the flask was lowered to room temperature (25 ℃), and tributyltin hydride (TBTH) was added to the flask at a rate of 1 ml/sec. After further stirring for 4 hours, distillation under reduced pressure at 1 Torr and 40 ° C yielded Nonafluorohexyltriethoxysilane (1H,1H,2H,2H-Perfluorohexyltriethoxysilane) in 75% yield.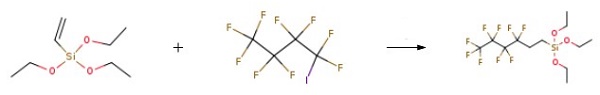
|
| | Nonafluorohexyltriethoxysilane Preparation Products And Raw materials |
|