1,4-dihydronicotinaMide riboside manufacturers
|
| | 1,4-dihydronicotinaMide riboside Basic information |
| Product Name: | 1,4-dihydronicotinaMide riboside | | Synonyms: | 1,4-dihydronicotinaMide riboside;1-(D-Ribo furanosyl)-1,4-dihydro-3-pyridine carboxamide;3-Pyridinecarboxamide, 1,4-dihydro-1-β-D-ribofuranosyl-;Reduced nicotinamide riboside;D-Ribofuranosyldihydronicotinamide, mixture of isomers;1-((2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1,4-dihydropyridine-3-carboxamide;1-(β-D-Ribofuranosyl)-1,4-dihydronicotinamide;1,4-Dihydro-1-β-D-ribofuranosyl-3-pyridinecarboxamide | | CAS: | 19132-12-8 | | MF: | C11H16N2O5 | | MW: | 256.26 | | EINECS: | | | Product Categories: | | | Mol File: | 19132-12-8.mol | 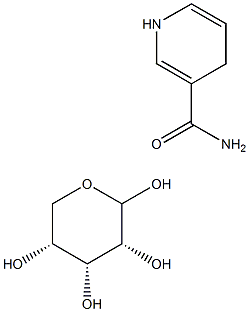 |
| | 1,4-dihydronicotinaMide riboside Chemical Properties |
| Boiling point | 598.3±50.0 °C(Predicted) | | density | 1.539±0.06 g/cm3(Predicted) | | pka | 13.37±0.70(Predicted) | | InChI | InChI=1/C6H8N2O.C5H10O5/c7-6(9)5-2-1-3-8-4-5;6-2-1-10-5(9)4(8)3(2)7/h1,3-4,8H,2H2,(H2,7,9);2-9H,1H2/t;2-,3-,4-,5?/s3 | | InChIKey | HMERANLWHIIDCI-UCPMJXAVNA-N | | SMILES | C(N)(=O)C1=CNC=CC1.OC1OC[C@@H](O)[C@@H](O)[C@H]1O |&1:13,15,17,r| |
| | 1,4-dihydronicotinaMide riboside Usage And Synthesis |
| Uses | Reduced Nicotinamide Riboside is a precursor which is an essential cofactor and substrate for many cells. | | Definition | ChEBI: 1-(beta-D-ribofuranosyl)-1,4-dihydronicotinamide is a pyridine nucleoside consisting of 1,4-dihydronicotinamide with a beta-D-ribofuranosyl moiety at the 1-position. It is a dihydropyridine and a pyridine nucleoside. | | Preparation |
There are very few methods for preparing 1,4-dihydronicotinaMide riboside (NRH). This compound can be prepared from dihydronicotinamide mononucleotide (NMNH) by hydrolysis of the 5′-phosphate ester in alkaline phosphatase. This method is time-consuming and is not cost-effective because NMNH as a precursor must be enzymatically hydrolyzed from NADH. Another method for synthesising NRH is reducing NR in the presence of sodium dithionate (Na2S2O4) as a reducing agent. In this method, nicotinamide riboside triflate is reduced to NRH in an aqueous solution of sodium dithionate and potassium hydrogen phosphate. Because the aqueous solution of Na2S2O4 is very unstable at ambient conditions, this reaction must be carried out at low temperatures and under anaerobic, alkaline conditions[1].
| | Biological Activity | 1,4-dihydronicotinamide riboside (NRH) is a potent NAD+ concentration enhancer both in vitro and in vivo. Researchers found that, after administration of NRH to mammalian cells, the NAD+ concentration increased by 2.5–10fold over control values in just one hour. Their findings demonstrate that the use of NRH is more effective than either NR or NMN. Moreover, NRH considerably enhances the NAD+/NADH ratio in the cultured cells without induction of apoptotic markers or a substantial increase in cell lactate levels. More recently, Canto et al. have found that contrary to the NR pathway, NRH uses different steps and enzymes to synthesize NAD+. That explains why NRH is a more effective and faster NAD+ precursor than NR in mammalian cells. In experiments with mice, the same researchers have also demonstrated that NRH is orally bioavailable as an NAD+ precursor and prevents cisplatin-induced acute kidney injury. In addition to increasing NAD+ levels, NRH can also deplete some genotoxins, such as hydrogen peroxide and methylmethane sulfonate. As a result, the mouse cells treated with NRH are resistant to cell death[1].
| | References | [1] Zarei, Amin et al. “Dihydronicotinamide riboside: synthesis from nicotinamide riboside chloride, purification and stability studies?.” RSC Advances 34 (2021): 21036–21047. |
| | 1,4-dihydronicotinaMide riboside Preparation Products And Raw materials |
|