- Ethionamide
-

- $100.00 / 1KG
-
2025-03-05
- CAS:536-33-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5000KG
- Ethionamide
-

- $0.00 / 25kg
-
2025-03-03
- CAS:536-33-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- Ethionamide
-

- $0.00 / 25kg
-
2025-03-03
- CAS:536-33-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
Related articles - Mechanism of action of Ethionamide
- Ethionamide (2-ethyl-4-pyridinecarbothioamide) is a derivative of isonicotinic acid that was first synthesized in France in 19....
- Mar 29,2022
|
| | Ethionamide Basic information |
| | Ethionamide Chemical Properties |
| Melting point | 164 °C | | Boiling point | 167 °C / 1mmHg | | density | 1.1332 (rough estimate) | | refractive index | 1.5500 (estimate) | | Fp | >110°(230°F) | | storage temp. | 2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | pKa 4.37(H2O t=25.0 I=0.025) (Uncertain) | | color | Yellow | | Water Solubility | Soluble in DMSO. Sparingly soluble in water | | Merck | 14,3737 | | BCS Class | 3/1 | | InChIKey | AEOCXXJPGCBFJA-UHFFFAOYSA-N | | CAS DataBase Reference | 536-33-4(CAS DataBase Reference) | | IARC | 3 (Vol. 13, Sup 7) 1987 | | NIST Chemistry Reference | Ethionamide(536-33-4) | | EPA Substance Registry System | Ethionamide (536-33-4) |
| | Ethionamide Usage And Synthesis |
| Description | Ethionamide is an antimycobacterial compound that is active against M. tuberculosis (MICs = 0.3-1.25 μg/ml). It is activated via oxidation by flavin monooxygenase and inhibits the InhA enzyme involved in mycolic acid biosynthesis. Formulations containing ethionamide have been used in the second-line treatment of multi-drug resistant tuberculosis. | | Chemical Properties | Yellow Solid | | Originator | Trecator,Theraplix,France,1959 | | Uses | Antibacterial (tuberculostatic). | | Uses | Ethionamide is used in antimicrobials and in potency assay of test compounds on M. tuberculosis. | | Definition | ChEBI: A thiocarboxamide that is pyridine-4-carbothioamide substituted by an ethyl group at position 2. A prodrug that undergoes metabolic activation by conversion to the corresponding S-oxide. | | Indications | Ethionamide (Trecator) is a derivative of isonicotinic
acid and is chemically related to isoniazid. It is a secondary
agent used in combination when primary agents
are ineffective or contraindicated; it is a bacteriostatic
antituberculosis agent. Its exact mechanism of action
is unknown but is believed to involve inhibition of
oxygen-dependent mycolic acid synthesis. It is thought
that mutations in the region of the (inhA) gene that are
involved in mycolic acid synthesis can cause both isoniazid
and ethionamide resistance. | | Manufacturing Process | Ethyl Propionyl-Pyruvate: 36 grams of methyl ethyl ketone and 73 grams of
ethyl oxalate are condensed in the presence of sodium ethylate, the reaction
mixture being refluxed in an alcoholic medium. 28 grams of the desired
product having a boiling point of 100° to 105°C/6 mm are obtained.
3-Cyano-4-Carbethoxy-6-Ethyl-2-Pyridone: 205 cc of 60% alcohol, 22 grams
of the product just obtained, 11 grams of cyanacetamide and 4.5 cc of
piperidine are refluxed. 19 grams of product having a melting point of 211°C
are obtained.
4-Carboxy-6-Ethyl-2-Pyridone: 30 grams of the cyanopyridone just obtained
are refluxed with concentrated hydrochloric acid. 13.5 grams of product
having a melting point of 308°C are obtained.
2-Chloro-4-Carbethoxy-6-Ethyl-Pyridine: 26 grams of the product just
obtained are treated with 81 grams of phosphorus pentachloride in 45 cc of
phosphorus oxychloride. The phosphorus oxychloride is distilled off in a
vacuum and the residue is treated with absolute alcohol. After distillation
there are obtained 24 grams of product having a boiling point of 127° to
131°C/8 mm.
Ethyl-2-Ethyl-Isonicotinate: 10 grams of the ester just obtained dissolved in
80 cc of absolute alcohol containing 5.5 grams of potassium acetate are
hydrogenated catalytically on 5% palladium black. 8 grams of product having
a boiling point of 120° to 124°C/14 mm are obtained.
2-Ethyl-Isonicotinic-Amide: 20 grams of the ether just obtained are agitated,
with 25 cc of concentrated ammonia. 11 grams of product having a melting
point of 131°C are obtained.
2-Ethyl-Isonicotinic Nitrile: The 11 grams of the amide just obtained are
treated with 15 grams of phosphorus anhydride at 160° to 180°C in a
vacuum. 6 grams of a liquid residue are obtained.
Alpha-Ethyl-Isonicotinic Thioamide: The 6 grams of the liquid just obtained, in
solution in 15 cc of absolute alcohol containing 2 grams of triethanolamine,
are treated with hydrogen sulfide. 6.5 grams of the desired product having a
melting point of 166°C are obtained. | | Brand name | Trecator (Wyeth. | | Therapeutic Function | Antitubercular | | General Description | Yellow crystals or canary yellow powder with a faint to moderate sulfide odor. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | A thiocarbamate/amine. Thiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides. | | Fire Hazard | Flash point data for Ethinamide are not available. Ethinamide is probably combustible. | | Biochem/physiol Actions | Ethionamide is used as an anti-tuberculosis antibiotic and an inducer of hypothyroidism. | | Mechanism of action | Evidence has been presented suggesting that the mechanism of action of ethionamide is similar to that of INH. Similar to INH, ethionamide is considered to be a pro-drug, which is converted via oxidation by catalase-peroxidase to an active acylating agent, ethionamide sulfoxide, which in turn inactivates the inhA enoyl reductase enzyme. In the case of ethionamide, it has been proposed that the ethionamide sulfoxide acylates Cys-243 in inhA protein. | | Pharmacology | Ethionamide is well absorbed following oral administration.
It is rapidly and widely distributed to all body
tissues and fluids, including the cerebrospinal fluid.
Metabolism of ethionamide is extensive, and several dihydropyridine
metabolites are produced. Less than 1%
of the drug is eliminated in the urine unchanged.
GI disturbances, including nausea, vomiting, and intense
gastric irritation, are frequent. In addition, ethionamide
may cause a wide range of neurological side
effects, such as confusion, peripheral neuropathy, psychosis,
and seizures. Neurological effects can be minimized
by pyridoxine supplementation. Other rare side
effects include gynecomastia, impotence, postural hypotension,
and menorrhagia. | | Clinical Use | 2-Ethylthioisonicotinamide (Trecator SC) occurs as a yellowcrystalline material that is sparingly soluble in water. Thisnicotinamide has weak bacteriostatic activity in vitro but, becauseof its lipid solubility, is effective in vivo. In contrast tothe isoniazid series, 2-substitution enhances activity in thethioisonicotinamide series.
Ethionamide is rapidly and completely absorbed followingoral administration. It is widely distributed throughoutthe body and extensively metabolized to predominantly inactiveforms that are excreted in the urine. Less than 1% ofthe parent drug appears in the urine.Ethionamide is considered a secondary drug for the treatmentof tuberculosis. It is used in the treatment of isoniazidresistanttuberculosis or when the patient is intolerant toisoniazid and other drugs. Because of its low potency, thehighest tolerated dose of ethionamide is usually recommended.Gastrointestinal intolerance is the most commonside effect associated with its use. Visual disturbances andhepatotoxicity have also been reported. | | Synthesis | Ethionamide, 2-(ethyl)isonicotinthioamide (34.1.18), a derivative of isoni�cotinic acid, is synthesized by the following scheme. Diethyl oxalate is condensated with
methylethylketone in the presence of sodium ethoxide to form the ethyl ester of propi�onylpyruvic acid (34.1.12). Condensation of this with cyanoacetamide results in heterocy�clization, to form 3-cyano-4-carboethoxy-6-ethyl-2-pyridone (34.1.13), which is
hydrolyzed with hydrochloric acid to give 4-carboxy-6-ethyl-2-pyridone (34.1.14).
Reacting this with a mixture of phosphorous oxychloride and pentachloride gives 6-ethyl-
2-chloroisonicotinic acid chloride, which is subsequently treated with ethyl alcohol to
obtain the ethyl ester of 6-ethyl-2-chloroisonicotinic acid (34.1.15). Reducing this with
hydrogen over a palladium catalyst removes the chlorine atom at position 2 of the pyridine
ring, giving the ethyl ester of 6-ethylisonicotinic acid (34.1.15). Interacting this with
ammonia, followed by dehydration of the resulting amide of 6-ethylisonicotinic acid using
phosphorous pentoxide gives the nitrile of 6-ethylisonicotinic acid (34.1.17). Finally,
reacting this with hydrogen sulfide gives ethionamide. 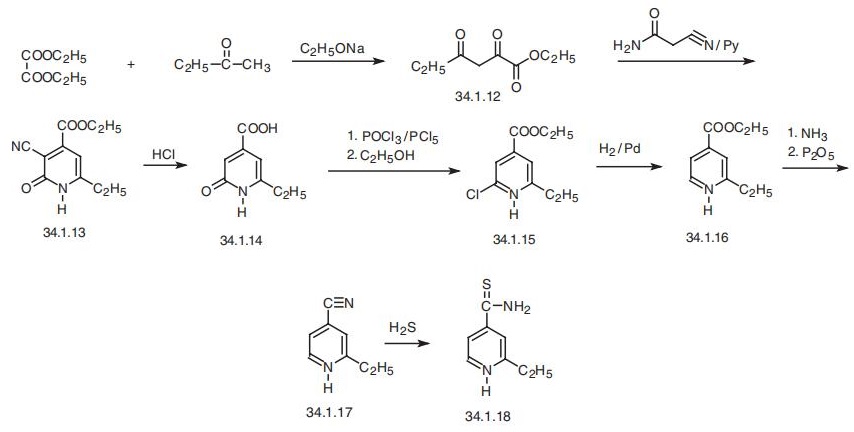
| | Metabolism | Ethionamide is orally active but is not well tolerated in a single large dose (>500 mg). The GI irritation can be reduced by administration with meals. Additional side effects may include central nervous system (CNS) effects, hepatitis, and hypersensitivities. Less than 1% of the drug is excreted in the free form, with the remainder of the drug appearing as one of six metabolites. Among the metabolites are ethionamide sulfoxide, 2-ethylisonicotinamide, and the N-methylated- 6-oxodihydropyridines. | | Purification Methods | It crystallises from EtOH as lemon yellow needles. The hydrochloride crystallises from EtOH (+ few drops of HCl) as orange yellow needles with m 212-214o. [Kutscherowa et al. J Gen Chem USSR (English transl) 29 915 1959, Beilstein 22 III/IV 737.] It causes peripheral and occular neuropathy and is carcinogenic and teratogenic. |
| | Ethionamide Preparation Products And Raw materials |
|