|
|
| | Tenofovir alafenamide hemifumarate Basic information |
| Product Name: | Tenofovir alafenamide hemifumarate | | Synonyms: | GS-7340 (hemifumarate);Anti-HIV;API-Anti-HBV;(S)-Isopropyl 2-(((S)-((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl)(phenoxy)phosphoryl)amino)propanoate fumarate(2:1);(R)-isopropyl 2-(((S)-((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl)(phenoxy)phosphoryl)amino)propanoate fumarate;N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester;Tenofovir alafenamide hemifumarate;TENOFOVIR ALAFENAMIDE HEMIFUMARATE (GS-7340 HEMIFUMARATE) | | CAS: | 1392275-56-7 | | MF: | C25H33N6O9P | | MW: | 592.55 | | EINECS: | 805-448-8 | | Product Categories: | API;1392275-56-7 | | Mol File: | 1392275-56-7.mol | 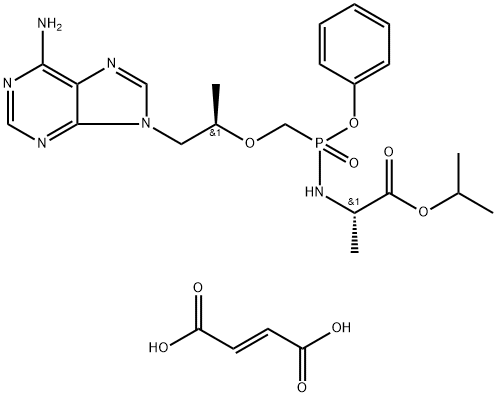 |
| | Tenofovir alafenamide hemifumarate Chemical Properties |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | DMSO:75.0(Max Conc. mg/mL);70.16(Max Conc. mM) | | form | Solid | | color | White to off-white | | CAS DataBase Reference | 1392275-56-7 |
| | Tenofovir alafenamide hemifumarate Usage And Synthesis |
| Description | Tenofovir alafenamide hemifumarate is an investigational oral prodrug of Tenofovir. Tenofovir is a HIV-1 nucleotide reverse transcriptase inhibitor. | | Chemical Properties | White to almost White crystal or crystalline powder. | | Originator | Gilead Sciences | | Uses | Tenofovir alafenamide hemifumarate is a newer, more effective prodrug used in combination with FTC that has recently been approved for the prevention of HIV transmission through the rectum. It is used to treat chronic hepatitis B virus (HBV) infection in adults with compensated liver disease. | | Definition | ChEBI: Tenofovir alafenamide fumarate is a fumarate salt prepared from tenofovir alafenamide by reaction of one molecule of fumaric acid for every two molecules of tenofovir alafenamide. A prodrug for tenofovir, it is used in combination therapy for the treatment of HIV-1 infection. It has a role as an antiviral drug, a HIV-1 reverse transcriptase inhibitor and a prodrug. It contains a tenofovir alafenamide(1+). | | Clinical Use | Tenofovir alafenamide hemifumarate is useful in the treatment and/or prophylaxis of one or more viral infections in man or animals, including infections caused by DNA viruses. RNA viruses, herpesviruses (e.g., CMV, HSV 1, HSV 2, VZV), retroviruses, hepadnaviruses (e.g., HBV), papillomavirus, hantavirus, adenoviruses and HIV. Like tenofovir disoproxil, tenofovir alafenamide is another prodrug form of tenofovir, and can be used in the treatment and/or prophylaxis of the same conditions. | | Side effects | Check with your doctor immediately if any of the following side effects occur:
Abdominal or stomach discomfort,bloody urine,dark urine,decreased appetite,decreased frequency or amount of urine,troubled breathing,cough,headache,back pain. | | Synthesis | Under a nitrogen atmosphere, 200 g of isopropanol, 9 g of fumaric acid, and 50 g of TAF-3 were sequentially added to a 2 L reaction flask. Turn on the stirring, control the temperature at 40 ~ 50 °C, stir and dissolve, filter while hot, collect the mother liquor. Transfer the mother liquor to a 2L reaction bottle, start stirring, control the temperature at 40 ~ 50 °C, stir and dissolve, slowly reduce the temperature to 0 ~ 5 ° C, cooling time 2 ~ 3 hours, heat stirring for 10 hours. Filtration, drying at 60-65 ° C for 12 to 16 hours, to obtain 37.5 g of Tenofovir Alafenamide Fumarate finished product, white powder, yield 75.0%.
| | Drug interactions | Some products that may interact with this drug are: adefovir, orlistat, other drugs that may harm the kidneys (including aminoglycosides such as amikacin/gentamicin).
| | Advantages | One major advantage of the hemifumarate form of tenofovir alafenamide over the monofumarate form is its exceptional capability to purge GS-7339 (i.e., 9-[(R)-2-[[(R)-[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]propyl]adenine, which is the major diastereomeric impurity in the active pharmaceutical ingredient. Thus, the hemifumarate form of tenofovir alafenamide can be more readily and easily separated from impurities than the monofumarate form. Other major advantages of tenofovir alafenamide hemifumarate over the monofumarate form include improved thermodynamic and chemical stability (including long-term storage stability), superior process reproducibility, superior drug product content uniformity, and a higher melting point. |
| | Tenofovir alafenamide hemifumarate Preparation Products And Raw materials |
|