| Company Name: |
S.Z. PhyStandard Bio-Tech. Co., Ltd.
|
| Tel: |
0755-4000505016 13380397412 |
| Email: |
3001272453@qq.com |
| Products Intro: |
Product Name:Cabozantinib Impurity 6
CAS:1621681-63-7
Purity:98% HPLC Package:10mg;50mg;100mg
|
| Company Name: |
Beijing Comparison Pharmaceutical Technology Co., Ltd
|
| Tel: |
15801002657 15701683040 |
| Email: |
sales@bjcomparison.com |
| Products Intro: |
Product Name:4-(4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamido)phenoxy)-6,7-dimethoxyquinoline 1-oxide
CAS:1621681-63-7
Purity:≥95% Package:100mg/;50mg/;25mg/;10mg/
|
| Company Name: |
Shanghai Kewel Chemical Co., Ltd.
|
| Tel: |
021-64609169 18901607656 |
| Email: |
greensnown@163.com |
| Products Intro: |
Product Name:Cabozantinib Impurity 6
CAS:1621681-63-7
Purity:95+ HPLC; Package:10mg;25mg;50mg;100mg
|
| Company Name: |
Shenzhen Botel Biotechnology Co. Ltd.
|
| Tel: |
0755-22202135 13316968096 |
| Email: |
1979313431@qq.com |
| Products Intro: |
Product Name:Cabozantinib Impurity 8
CAS:1621681-63-7
Purity:98% HPLC Package:10mg;25mg;50mg
|
|
| | Cabozantinib impurity 1DYH Basic information |
| Product Name: | Cabozantinib impurity 1DYH | | Synonyms: | N-[4-[(6,7-Dimethoxy-1-oxido-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-1,1-cyclopropanedicarboxamide;Cabozantinib impurity 1DYH;1,1-Cyclopropanedicarboxamide, N-[4-[(6,7-dimethoxy-1-oxido-4-quinolinyl)oxy]phenyl]-N'-(4-fluorophenyl)-;4-(4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamido)phenoxy)-6,7-dimethoxyquinoline 1-oxide ;N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)phenyl)-6,7-dimethoxyquinolin-4-amine;Cabozantinib Impurity 04 | | CAS: | 1621681-63-7 | | MF: | C28H24FN3O6 | | MW: | 517.51 | | EINECS: | | | Product Categories: | | | Mol File: | 1621681-63-7.mol | 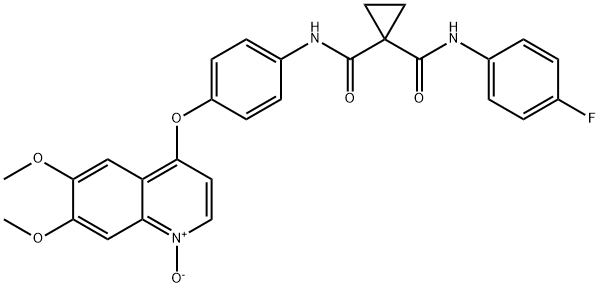 |
| | Cabozantinib impurity 1DYH Chemical Properties |
| density | 1.36±0.1 g/cm3(Predicted) | | pka | 13.86±0.70(Predicted) |
| | Cabozantinib impurity 1DYH Usage And Synthesis |
| Uses | Cabozantinib N-oxide is a impurity of Cabozantinib (X745500), a medication used to treat medullary thyroid cancer. As well, it can be used in biological study. Computational network biological approach based on pathway cross-talk inhibition identified new synergistic drug combinations using raloxifene and cabozantinib for treatment of human breast cancer in xenograft mouse model. Potent c-MET inhibitor. |
| | Cabozantinib impurity 1DYH Preparation Products And Raw materials |
|