- Imipramine
-

- $52.00 / 5mg
-
2024-11-11
- CAS:50-49-7
- Min. Order:
- Purity: 99.4%
- Supply Ability: 10g
|
| | IMIPRAMINE Basic information |
| Product Name: | IMIPRAMINE | | Synonyms: | IM;Imidobenzyle;Imipramina;Imiprin;Imizin;Imizine;Imizinum;Impramine | | CAS: | 50-49-7 | | MF: | C19H24N2 | | MW: | 280.41 | | EINECS: | 200-042-1 | | Product Categories: | TOFRANIL | | Mol File: | 50-49-7.mol | 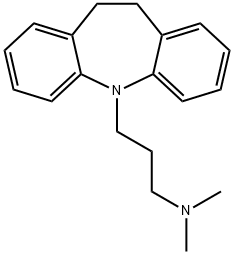 |
| | IMIPRAMINE Chemical Properties |
| Melting point | 174°C | | Boiling point | bp0.1 160° | | density | 0.9935 (rough estimate) | | refractive index | 1.5640 (estimate) | | pka | pKa 9.66(H2O,t = 25,I=0.025) (Uncertain) | | form | Liquid | | color | Colorless to light yellow | | Water Solubility | 18.23mg/L(24 ºC) | | Solvent | Ethanol under nitrogen | | Concentration | 1 mCi/ml | | Specific Activity | 60-90 Ci/mmol | | BCS Class | 1 | | EPA Substance Registry System | Imipramine (50-49-7) |
| Hazardous Substances Data | 50-49-7(Hazardous Substances Data) | | Toxicity | A tertiary amine tricyclic antidepressant that is thought
to exert its therapeutic effect by inhibiting the reuptake of serotonin
and norepinephrine centrally. A major metabolite is N-desmethylimipramine
(desipramine), also used as an antidepressant
drug. Desipramine differs from imipramine in being a better
blocker of norepinephrine, rather than serotonin, uptake. Side
effects, including sedation and drowsiness, dry mouth, urinary
retention, constipation, and orthostatic hypotension, are probably
due to the anticholinergic, anti-α-adrenergic, and antihistaminergic
receptor-blocking properties. Imipramine should not be used
in conjunction with a monoamine oxidase inhibitor or other
treatment that increases catecholamine concentrations (e.g.,
drugs containing sympathomimetic amines). Imipramine should
be avoided in patients with cardiovascular disease or seizure disorder,
or in those who may abuse alcohol, as imipramine lowers
seizure threshold, can produce cardiovascular toxicity and may
potentiate the effects of alcohol. Imipramine intoxication can
include CNS abnormalities (e.g., drowsiness, stupor, coma, and
extrapyramidal symptoms), cardiac arrhythmia, and respiratory
depression. Children appear to be particularly vulnerable to the
cardiotoxic and seizure-inducing effects of high doses of imipramine.
The oral LD50 in female rats is 305 mg/kg. |
| | IMIPRAMINE Usage And Synthesis |
| Originator | Tofranil,Ciba Geigy,France,1959 | | Uses | Imipramine is used in depression of various etiology accompanied by motor
clumsiness and enuresis in children and Parkinson’s disease. | | Uses | antidepressant | | Definition | ChEBI: Imipramine is a dibenzoazepine that is 5H-dibenzo[b,f]azepine substituted by a 3-(dimethylamino)propyl group at the nitrogen atom. It has a role as an adrenergic uptake inhibitor, an EC 3.4.21.26 (prolyl oligopeptidase) inhibitor and an antidepressant. It derives from a hydride of a 5H-dibenzo[b,f]azepine. | | Manufacturing Process | 20 parts of imino dibenzyl are dissolved in 100 parts by volume of absolutely
dry benzene. A suspension of 4 parts NaNH2 in 50 parts by volume of
absolute benzene are then added dropwise at 50° to 60°C after which the
mixture is boiled for an hour under reflux. 13 parts of 3-dimethylamino n_x0002_propyl chloride are then added dropwise at 40° to 50°C and the mixture is
boiled for 10 hours under reflux. After cooling, the benzene solution is
thoroughly washed with water, whereupon the basic constituents are extracted
with dilute hydrochloric acid.
The hydrochloric extract is then made alkaline and the separated base is
extracted with ether. After drying, the solvent is evaporated and the residue is
distilled in the high vacuum, whereby the N-(3-dimethylaminopropyl)-imino
dibenzyl passes over at a temperature of 160°C under 0.1 mm pressure. The
chlorohydrate with a melting point of 174° to 175°C is obtained therefrom
with alcoholic hydrochloric acid. | | Brand name | Janimine (Abbott);
Pramine (Alra); Presamine (Sanofi Aventis); Tofranil
(Novartis); Tofranil (Tyco). | | Therapeutic Function | Antidepressant | | Mechanism of action | Besides being used in the clinical treatment of depression, imipramine also has been used for the treatment
of functional enuresis in children who are at least 6 years of age (25 mg daily administered 1 hour before
bedtime, not to exceed 2.5 mg/kg daily). | | Clinical Use | Imipramine is a 10,11-dihydrodibenzazepine tertiary amine TCA that is marketed as hydrochloride
and pamoate salts, both of which are administered orally. Although the hydrochloride salt may be
administered in divided daily doses, imipramine's long duration of action suggests that the entire oral daily
dose may be administered at one time.On the other hand, imipramine pamoate usually is administered as a
single daily oral dose. | | Synthesis | Imipramine, 5-[3-(dimethylamino)propyl]-10,11-dihydro-5H-dibenz[b,f]
azepine (7.1.1), is synthesized by the alkylation of 10,11-dihydro-5H-dibenz[b,f]azepine
using 3-dimethylaminopropylchloride in the presence of sodium amide [1¨C3]. 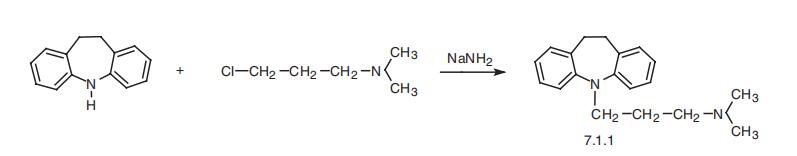
|
| | IMIPRAMINE Preparation Products And Raw materials |
|