| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
15002134094 |
| Email: |
marketing@targetmol.cn |
| Products Intro: |
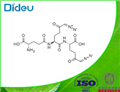
Product Name:Azotomycin
CAS:7644-67-9
Package:50mg/RMB 22800;100mg/RMB 29500;25mg/RMB 17200
|
Diazomycin B manufacturers
- Diazomycin B USP/EP/BP
-
- $1.10 / 1g
-
2021-07-02
- CAS:7644-67-9
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
|
| | Diazomycin B Basic information |
| Product Name: | Diazomycin B | | Synonyms: | Azotomycin;Azotomycin sodium salt;Diazomycin B;DuazomycinB;6-Diazo-N-(6-diazo-N-L-γ-glutamyl-5-oxo-L-norleucyl)-5-oxo-L-norleucine;Antibiotic-1719;NSC-56654;Antibiotic produced by streptomyces ambofaciens | | CAS: | 7644-67-9 | | MF: | C17H23N7O8 | | MW: | 453.41 | | EINECS: | | | Product Categories: | | | Mol File: | 7644-67-9.mol |  |
| | Diazomycin B Chemical Properties |
| Boiling point | 560.56°C (rough estimate) | | density | 1.2516 (rough estimate) | | refractive index | 1.6800 (estimate) |
| Toxicity | TDLo ivn-hmn: 192 mg/kg/4D:GIT CTRRDO 61,1719,77 |
| | Diazomycin B Usage And Synthesis |
| Originator | Azotomycin,ZYF Pharm | | Uses | Antineoplastic. | | Manufacturing Process | Azotomycin is anticancer antibiotic produced by Streptomyces ambofaciens.
Total sythesis of it from γ-benzyl-N-tert-butyloxycarbonyl-L-glutamic acid (γ-
OBzl-N-Boc-L-Glu) has been accomplished in nine steps. The mixed carbonic
anhydride method was chosen for peptide bond formation. Commerically
available γ-OBzl-N-Boc-L-Glu was esterified with ethereal diazomethane,
deprotected with trifluoroacetic acid-methylene chloride (1:1), and converted
to hydrochloride γ-benzyl-L-glutamic acid α-methyl ester (γ-OBzl-L-Glu-α-OMe
HCl) by treatment with dry hydrogen chloride in ethyl ether, MP: 129°-135°C
(dec.), [α]D
25= + 13.3° (CHCl3).
Reaction of γ-OBzl-N-Boc-L-Glu with isobutyl chloroformate and Nmethylmorpholine
followed by addition of γ-OBzl-L-Glu-α-OMe HCl afforded
dipeptide γ-OBzl-N-Boc-L-Glu-γ-OBzl-L-Glu-α-OMe, yield 93%, MP: 58.5°-
60°C, [α]D
25= + 7.10° (CHCl3). After cleaving (trifluoroacetic acid-methylene
chloride) the Boc group of above dipeptide, N-Tfa-L-Glu-OMe (Tfatrifluoroacetyl)
was condensed with the product to afford tripeptide N-(γ-NTfa-
L-Glu-α-OMe)-v-OBzl-N-Boc-L-Glu-γ-OBzl-L-Glu-α-OMe as colorless
crystals, MP: 120°-122°C, [α]D
25= + 7.7° (CHCl3). Following hydrogenolysis
of benzyl esters using palladium-on-carbon (10%), diacid N-(γ-N-Tfa-L-Glu-α-
OMe)-L-Glu-L-GluOMe was obtained in yield 94% as colorless crystals, MP:
181°-184°C, [α]D
25= - 37.6° (CH3OH).
Selectively protected above diacid was converted to (γ-N-Tfa-L-Glu-α-OMe)-LGlu-
L-Glu-tetra-OMe next way. To a solution of N-(γ-N-Tfa-L-Glu-α-OMe)-L-Glu-
L-Glu-α-OMe (0.18 g) in dry acetone (20 ml cooled to 0°C was added with
magnetic stirring ethereal diazomethane (0.8 mole) until yellow color
persisted. After evaporation of solvent, the colorless solid was recrystallized
from methylene chloride-hexanes to afford 0.17 g (92%) of colorless crystals
of (γ-N-Tfa-L-Glu-α-OMe)-L-Glu-L-Glu-tetra-OMe, MP: 150°-152°C, [α]D
25= -
8.5° (CHCl3).
A 1.00 g (1.9 mmoles) of (γ-N-Tfa-L-Glu-α-OMe)-L-Glu-L-Glu-tetra-OMe was
dissolved under argon in dry dimethoxyethane (30 ml) with warming and
magnetic stirring, and triethylamine (0.55 ml, 3.97 mmoles) was added. The
reaction mixture was cooled to -30°C (dry ice isopropyl alcohol), and oxalyl
chloride (0.35 ml, 3.97 mmoles) was added followed by dimethylformamide (2drops). The reaction mixture was warmed to 0°C by addition of hot isopropyl
alcohol to the dry ice bath, stirred for 40 min, and then cooled to -78°C.
The cold acid chloride solution was slowly (30 min) added through the
sintered-glass filter into ethereal solution of diazomethane (0.5 moles, 30 ml)
cooled to -23°C (dry ice-carbon tetrachloride). After the mixture was stirred
30 min at -23°C and 30 min at 0°C, solvent was evaporated by steam argon,
and the residue was chromatographed in 19:1 ethyl acetate-methanol on
column of silica gel (70 g). The fraction (24:1 ethyl acetate-methanol) with
TLC Rf 0.16 were collected and solvent evaporated to 0.30 g (38 %) of light
yellow crystals of N-Tfa(-)-azotomycin-di-OMe, which upon recrystallization
from chloroform-ethyl ether, melted at 134°-136°C, [α]D
25= - 22.9°.
To a mixture of N-Tfa(-)-azotomycin-di-OMe (106 mg, 0.18 mmoles) and
methanol (0.12 ml) was added 1.0 N sodium hydroxide (0.83 ml, 8.3 mmoles)
at room temperature and stirred for 30 min. Then the solution was acidified to
pH 6.9 with 0.1 N hydrochloric acid and extracted with chloroform (2x25 ml).
The aqueous phase was dissolved in methanol and passed through a column
of Sephadex LH-20 (250 g), and fractions containing the magor product (TLC
Rf 0.31, methanol, tailing) were collected. Solvent was evaporated and the
residue was freeze-dried to yield 54.3 mg (65%) of (-)-azotomycin as a light
yellow solid, which slowly decomposed at ambient temperature, [α]D
25= -
4.3°, IR, UV, 1H NMR, 13C NMR spectrum confirmed the structures of all
described compounds. | | Therapeutic Function | Antineoplastic | | Safety Profile | Poison by ingestion,intraperitoneal, subcutaneous, and intravenous routes.Human gastrointestinal tract effects by intravenous route.When heated to decomposition it emits toxic fumes ofNOx. |
| | Diazomycin B Preparation Products And Raw materials |
|