| Company Name: |
Glenmark Pharmaceuticals Limited
|
| Tel: |
+912240189999 |
| Email: |
GlobalPV@glenmarkpharma.com |
| Products Intro: |
Product Name:Frovatriptan
CAS:158747-02-5
Purity:98% Package:1 kg,5 kg, 10 kg,25kg And 1MT
|
| Company Name: |
S.Z. PhyStandard Bio-Tech. Co., Ltd.
|
| Tel: |
0755-83725681-603 |
| Email: |
|
| Products Intro: |
Product Name:R-Frovatriptan
CAS:158747-02-5
Purity:98% HPLC Package:10mg/25mg/50mg/100mg
|
|
| | Frovatriptan Basic information |
| Product Name: | Frovatriptan | | Synonyms: | R-Frovatriptan;1H-Carbazole-6-carboxamide, 2,3,4,9-tetrahydro-3-(methylamino)-, (3R)-;1H-Carbazole-6-carboxamide, 2,3,4,9-tetrahydro-3-(methylamino)-, (R)-;Hsdb 7363;Sb 209509;Unii-H82Q2D5wa7;FROVATRIPTAN;Frovatriptan 13CD3 | | CAS: | 158747-02-5 | | MF: | C14H17N3O | | MW: | 243.3 | | EINECS: | | | Product Categories: | | | Mol File: | 158747-02-5.mol | 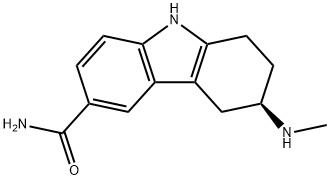 |
| | Frovatriptan Chemical Properties |
| Boiling point | 515.2±50.0 °C(Predicted) | | density | 1.27±0.1 g/cm3(Predicted) | | pka | 16.39±0.40(Predicted) |
| | Frovatriptan Usage And Synthesis |
| Originator | Frova,Elan Corporation | | Definition | ChEBI: Frovatriptan is a member of carbazoles. | | Manufacturing Process | 4-Carboxamidophenylhydrazine hydrochloride (2.87 g) and 4-
phthalimidocyclohexanone (3.00 g) were mixed in acetic acid and the mixture
was heated under reflux for 2 h. After cooling, the mixture was neutralized
using aq. potassium carbonate solution, and the yellow solid thus obtained
was filtered, washed with water, and dried. Purification by column
chromatography (SiO2; CHCl3/CH3OH) gave 6-carboxamido-3-phthalimido-
1,2,3,4-tetrahydrocarbazole (2.8 g).
The 6-carboxamido-3-phthalimido-1,2,3,4-tetrahydrocarbazole (1.0 g) was
suspended in ethanol (10 ml) and hydrazine hydrate (5 ml) was added. A
clear solution was obtained, and the mixture was left to stir overnight, to yield
a precipitate. The whole mixture was evaporated to dryness, washed with aq.
K2CO3 solution, and water, to leave the (+/-)-3-amino-6-carboxamido-1,2,3,4-
tetrahydrocarbazole (0.44 g), melting point 146°-148°C.
Separation of diastereoisomers of a chiral derivative of a 3-amino-6-
carboxamido-1,2,3,4-tetrahydrocarbazole e.g. by crystallisation, or by
chromatography.
Benzaldehyde (10.6 g) was added to a suspension of (+)-3-amino-6-
carboxamido-1,2,3,4-tetrahydrocarbazole (12.35 g) in methanol (100 ml). The
mixture was stirred for 1 h, sodium cyanoborohydride (9.3 g) added over 1 h
and the clear solution stirred for 24 h. The solution was cooled (ice bath) and
formaldehyde (37% aqueous methanolic, 9:1 solution, 5.5 ml) added. After
30 min stirring at room temperature water (100 ml) was added, stirring
continued for 30 min followed by extraction with dichloromethane (3 times
150 ml). The combined organic extracts were washed with water (2 times 200
ml), dried (Na2SO4), filtered and solvent removed at reduced pressure. The
residue was column chromatographed (silica gel, dichloromethane-10%
ethanol/dichloromethane) to give 3-N-benzyl-6-carboxamido-3-N�methylamino-1,2,3,4-tetrahydrocarbazole (9.4 g) as a foam. The succinate salt (1:1) was recrystallised from methanol, melting point 175°-182°C.
To a solution of 3-N-benzyl-6-carboxamido-3-N-methylamino-1,2,3,4-
tetrahydrocarbazole (1.0 g) in ethanol (100 ml) containing succinic acid (0.39
g), Pearlmans catalyst (1.0 g) was added and the mixture shaken under an
atmosphere of hydrogen at 45 psi and 50°C for 2 h. The mixture was filtered
(celite pad) and the pad washed thoroughly with ethanol. The combined
flitrate and washings were evaporated to dryness, coevaporated with ethanol
(3 times 100 ml) and recrystallised from methanol to give the (+)-6-
carboxamido-3-N-methylamino-1,2,3,4-tetrahydrocarbazole succinate (1:1)
salt, melting point 148°-155°C. | | Therapeutic Function | Migraine therapy | | Clinical Use | 5HT1 receptor agonist:
Acute relief of migraine | | Drug interactions | Potentially hazardous interactions with other drugs
Antidepressants: increased CNS toxicity with
citalopram - avoid; blood levels of frovatriptan
increased 27-49% by fluvoxamine - avoid; possibly
increased serotonergic effects with duloxetine,
venlafaxine and SSRIs; increased serotonergic effects
with St John’s wort - avoid.
Dapoxetine: possible increased risk of serotonergic
effects - avoid for 2 weeks after stopping 5HT1
agonists.
Ergot alkaloids: increased risk of vasospasm - avoid. | | Metabolism | Following oral administration of radiolabelled
frovatriptan, 32% of the dose was recovered in urine and
62% in faeces. Radiolabelled compounds excreted in
urine were unchanged frovatriptan, hydroxy frovatriptan,
N-acetyl desmethyl frovatriptan, hydroxy N-acetyl
desmethyl frovatriptan, and desmethyl frovatriptan,
together with several other minor metabolites formed
under the action of CYP1A2. Desmethyl frovatriptan
had about 3-fold lower affinity at 5-HT1 receptors than
the parent compound. N-acetyl desmethyl frovatriptan
had negligible affinity at 5-HT1 receptors. The activity of
other metabolites has not been studied. Renal clearance
accounted for 38% (82 mL/min) and 49% (65 mL/min)
of total clearance in males and females, respectively. |
| | Frovatriptan Preparation Products And Raw materials |
|