- Potassium carbonate
-

- $0.00 / 1kg
-
2025-04-15
- CAS:584-08-7
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000
- Potassium carbonate
-

- $99.00 / 1kg
-
2025-04-15
- CAS:584-08-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Potassium carbonate
-

- $3.50 / 1kg
-
2025-04-12
- CAS:584-08-7
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 3000tons/month
|
| Product Name: | Potassium carbonate | | Synonyms: | POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,FCC;POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,PURIFIED;POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,REAGENT,ACS;POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,REAGENT,ACS(BULK;POTASSIUMCARBONATE,ANHYDROUS,GRANULAR,USP;POTASSIUM CARBONATE: 99.998% PURATREM;Potassium carbonate,99+%,for analysis,anhydrous;Potassium Carbonate, Puratronic (Metals Basis) | | CAS: | 584-08-7 | | MF: | K2CO3 | | MW: | 138.21 | | EINECS: | 209-529-3 | | Product Categories: | Chemical Synthesis;Inorganic Bases;Materials Science;Metal and Ceramic Science;Potassium Salts;Synthetic Reagents;Inorganics;metal carbonate;FOOD ADDITIVES;INORGANIC & ORGANIC CHEMICALS;Analytical Reagents for General Use;O-P, Puriss p.a. ACS;Puriss p.a. ACS;Reagent Plus;Reagent Grade;Pharmacopoeia (USP);Pharmacopoeia A-ZPharmacopoeia (USP);Pharmacopoeial OrganicsEssential Chemicals;Routine Reagents;USP;ACS GradeEssential Chemicals;Adsorbents, Filter Aids and Drying Agents;Essential Chemicals;Other Drying Agents;584-08-7;Inorganic Bases;BioUltra Buffers;Biological Buffers;AlphabeticalChemical Synthesis;Puriss p.a.;O-P, Puriss p.a.;Synthetic Reagents;Salts;Potassium Salts;Inorganic BasesMetal and Ceramic Science;Chemical Synthesis | | Mol File: | 584-08-7.mol | 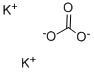 |
| | Potassium carbonate Chemical Properties |
| Melting point | 891 °C (lit.) | | Boiling point | decomposes [STR93] | | density | 2.43 g/mL at 25 °C | | bulk density | 750kg/m3 | | storage temp. | Store at +5°C to +30°C. | | solubility | H2O: 1 M at 20 °C, clear, colorless | | pka | 10.33[at 20 ℃] | | form | powder | | color | Yellow | | Specific Gravity | 2.29 | | PH | 10.52(1 mM solution);11(10 mM solution);11.36(100 mM solution); | | Odor | at 100.00?%. odorless | | Water Solubility | 1120 g/L (20 ºC) | | Sensitive | Hygroscopic | | λmax | λ: 260 nm Amax: 0.03
λ: 280 nm Amax: 0.02 | | Merck | 14,7619 | | BRN | 4267587 | | Dielectric constant | 5.6(16.0℃) | | Stability: | Stable. Incompatible with moisture, acids, magnesium bromine trifluoride and magnesium bromine trichloride. | | CAS DataBase Reference | 584-08-7(CAS DataBase Reference) | | NIST Chemistry Reference | Dipotassium carbonate(584-08-7) | | EPA Substance Registry System | Potassium carbonate (584-08-7) |
| | Potassium carbonate Usage And Synthesis |
| Description | Potassium carbonate (molecular formula: K2CO3), also known as potash or pearl ash, appears as a white powder or as colorless solid crystal with salty taste and deliquescence. It can be dissolved in water to form a strongly alkaline solution. However, it is insoluble in organic solvents such as ethanol. It has wide applications. For example, it can be used as a drying agent, buffering agent and a source of potassium in laboratory. It can also be used for the manufacturing of fire extinguishers, soap, glass, and soften water. In addition, it is also used during the production of cocoa powder to balance pH. Moreover, it can be supplied to effervescent tablets which can conveniently provide potassium when there are low levels of potassium in the blood for patients caused by various kinds of factors. In industry, potassium carbonate is manufactured though first performing electrolysis of potassium chloride to generate potassium hydroxide, followed by reaction with carbon dioxide to derive the product. It is toxic upon eye contact, inhalation and ingestion. For rats, it has an oral LD50 being 1870 mg/kg. It has chronic effects on humans, causing damage to the mucous membranes, skin, and eyes.

| | Chemical Properties | It belongs to monoclinic, and is white powder or granular crystal. It is easily soluble in water but insoluble in alcohol and ether. | | Uses | Potassium carbonate can serve as an effective alkaline agent and dough conditioner and is capable of hindering the fermentation process in noodles. It can be applied to pasta food, where its application quantity must be in accordance with production requirements. In addition, potassium carbonate can be used for the production of optical glass, as it enhances the transparency, strength, and refractive coefficient of the material. It is also useful as a welding electrode, preventing arc-breaking during welding. Further applications of potassium carbonate include its utility as a vat dye and as a white discharging agent for ice dyeing. It can be utilized as an absorbent for removing carbon dioxide and hydrogen sulfide, and quantities can be mixed with soda ash to create a dry powder extinguishing agent. | | Production method | The ion exchange method: dubbed 250 g/L solution and adding a small amount of potassium carbonate to remove calcium and magnesium ions. Ammonium bicarbonate solution is also prepared at a concentration 200 g/L in water. Pass the potassium chloride solution through a countercurrent into ion exchange column, to make the sodium resin R-Na become potassium type RK, wash off the chlorine ion within the gap of the soft resin; after finishing washing, put through the ammonium bicarbonate solution downstream into the resin exchange column, making the resin become ammonium type R-NH4 and obtain a mixed dilute solution of potassium bicarbonate and ammonium bicarbonate; go through evaporation once to decompose the ammonium bicarbonate; evaporate once again to decompose most of the potassium bicarbonate into potassium carbonate; the precipitated potassium chloride crystal after cooling was filtered and removed; further go through evaporation for three times to 54°C and filter to remove the compound salt of potassium and sodium. The solution was subject to carbonation to convert the potassium carbonate into potassium bicarbonate; further go through crystallization, separation, washing, calcination to obtain the finished product. Its reaction steps are as below:
R-Na + KCl → R-K + NaCl
R-K + NH4HCO3 → R-NH4 + KHCO3
2KHCO3 → K2CO3 + CO2 ↑+ H2O
Leblanc method: Mix the potassium, coal, limestone in a certain ratio, add water and stir, wherein the potassium chloride is maintained at 6% to 8%, the sodium salt is maintained in 8% to 10% and then subject to calcination at 900~1000 ℃ to obtain the black ash. The black ash is pulverized and subject to hot water extraction. The immersion is further subject to evaporation to precipitate some part of unconverted potassium sulfate out; it was further sent to the carbonation tower for pre-carbonation after separation. Add the potassium permanganate into the carbonation solution to remove the impurities precipitate of aluminum, silicon, and iron; the filtrate was evaporated to the precipitation of potassium carbonate, sodium compound salt and impurities; the clarifying solution further undergoes secondary carbonation to obtain the crude potassium carbonate crystals; it further goes through filtering, water washing, and further calcination at 500~600 ℃ to obtain the finished potassium carbonate product. Its reaction processes are:
K2SO4 + 2C + CaCO3 → K2CO3 + CaS + 2CO2 ↑
K2CO3 + CO2 + H2O → 2KHCO3
2KHCO3 → K2CO3 + CO2 ↑+ H2O
Recrystallization method: dissolve the technical grade potassium carbonate in distilled water for solution purification; remove the impurities through filtration; the filtrate is further subject to concentration by evaporation, cooling crystallization, centrifugation, and drying to obtain the finished product of anhydrous potassium carbonate.
| | Reference quality standards | Item Premium grade First grade Qualified II type
Potassium carbonate (K2CO3) content ≥ 99.0% 98.5% 96.0% 99.0%
Chloride (KCl) content ≤ 0.01% 0.10% 0.20% 0.03%
Sulfur compounds (in K2SO4) content ≤0.01% 0.10% 0.15% 0.04%
Iron (Fe) content of ≤ 0.001% 0.003% 0.010% 0.001%
Water-insoluble content ≤ 0.02% 0.05% 0.10% 0.04%
Burning loss ≤ 0.60% 1.00% 1.00% 0.80%
| | Content Analysis | To a pre-weighed weighing bottle with a plug, accurately weigh 1 g of dried sample measured according to the "loss on drying" assay and dissolve it in 50ml water. After adding of 2 drops of methyl red test solution (TS-149), stir continuously and titrate with 1mol/L hydrochloric acid titration until the solution turns into light pink. Heating the solution to boiling and further cool it down, continue to titrate to boiling until a pale pink color doesn’t disappears any more. Each Ml of 1mol/L hydrochloric acid is equivalent of 69.1 mg of potassium carbonate (K2CO3)
| | Toxicity | ADI does not make special provision (FAO/WHO, 2001).
LD50: 18.70mg/kg (rat, oral).
GRAS (FDA, §184.1619, 2000);
| | Chemical Properties | Potassium carbonate is a white, crystalline, salt that forms basic aqueous solutions used in the production of fertilizer, glass, ceramics, explosives, soaps, chemicals, and wool treatments. It was the main compound once referred to as potash, although the term today is not reserved exclusively for potassium carbonate, but for several potassium salts. In the fertilizer industry, potash refers to potassium oxide, K2O, rather than potassium carbonate. Pearlash is a purer form of potash made by heating potash to remove impurities. | | Uses | Potassium carbonate is used in the chemical industry as a source of inorganic potassium salts (potassium silicates, potassium bicarbonate), which are used in fertilizers, soaps, adhesives, dehydrating agents, dyes, and pharmaceuticals. Potassium carbonate used to make potassium lye produces soft soaps, which are liquids or semisolids rather than solids. Other uses of potassium carbonate includes use as a fire suppressant in extinguishers, as a CO2 absorbent for chemical processes and pollution control, an antioxidant in rubber additives, and in pharmaceutical formulations. | | Production Methods | Potassium carbonate can be obtained from ash or by electrolysis of potassium chloride. Another method involves reacting potassium hydroxide with carbon dioxide. The resulting liquid carbonate contains approximately 50% potassium carbonate in water. However, the solid product, which contains over 70% potassium carbonate, is expensive and only suitable for certain types of acidic soil. Additionally, neutralizing caustic potash with carbon dioxide also yields potassium carbonate. | | Definition | ChEBI: Potassium carbonate is a potassium salt that is the dipotassium salt of carbonic acid. It has a role as a catalyst, a fertilizer and a flame retardant. It is a carbonate salt and a potassium salt. | | General Description | Potassium carbonate (K2CO3) is a white salt, soluble in water (insoluble in ethanol) which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It presents a large capacity to absorb moisture.Corrosive to metals and tissue. Density 12.8 lb /gal. Used to make soaps, other potassium compounds, in liquid fertilizers. | | Air & Water Reactions | Water soluble. Addition of water evolves heat. | | Reactivity Profile | Potassium carbonate neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995]. | | Hazard | Solutions irritating to tissue. | | Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. | | Agricultural Uses | Potassium carbonate is used in agriculture and food production. Potassium carbonate is used as a spray or drip fertilizer and also as a constituent of compound fertilizers. Its high water solubility and alkaline property make it useful for supplying potassium to acidic soils, especially in vineyards and orchards. Dutch-processed cocoa uses potassium carbonate as an alkalizing agent to neutralize the natural acidity of cocoa. It is used to produce food additives like potassium sorbate and monopotassium phosphate. | | Industrial uses | Potassium carbonate is used for numerous applications. Its primary use is in the production of specialty glasses and ceramics. It is used to make optical glass, glass used for video screens in televisions and computers, and laboratory glassware. Its is used in certain glasses rather then cheaper sodium carbonate owing to its better compatibility with lead, barium, and strontium oxides incorporated in these glasses. These oxides lower the melting point of glass and produce a softer glass. Potassium carbonate has a higher refractive index than sodium carbonate producing a more brilliant glass. Potassium carbonate is a common flux combined with titanium dioxide to produce frits used in ceramics. A frit is a calcined mixture of fine silica, a pigment, and a flux that is ground a specific particle size and used to produce glazes, enamels, and additives in glass making. | | Biological Activity | Potassium carbonate(K2CO3) is used as a drying agent for ketones, alcohols, amines, and non-acidic compounds before distillation. It serves as a buffering agent and controls pH during wine and mead production. Potassium carbonate is also used in the extraction of artemisinin, a potent antimalarial drug, from Artemisia annua plant extracts. | | Safety Profile | Poison by ingestion. A
strong caustic. Incompatible with KCO,
chlorine trifluoride, magnesium. Mutation
data reported. When heated to
decomposition it emits toxic fumes of K2O. | | Purification Methods | It crystallises from water between 100o and 0o. The solubility in H2O is 105% at 0o, 127% at 60o and 205% at 135o (b of saturated solution). After two recrystallisations of technical grade material, it had B, Li and Fe at 1.0, 0.04 and 0.01 ppm, respectvely. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963.] | | References | https://en.wikipedia.org/wiki/Potassium_carbonate
https://www.drugs.com/inactive/potassium-carbonate-106.html
http://www.sciencelab.com/msds.php?msdsId=9926681
https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-Carbonate
Solubility of Potassium Carbonate and Potassium Hydrocarbonate in Methanol DOI:10.1021/JE020012V |
| | Potassium carbonate Preparation Products And Raw materials |
|